Figure 3.
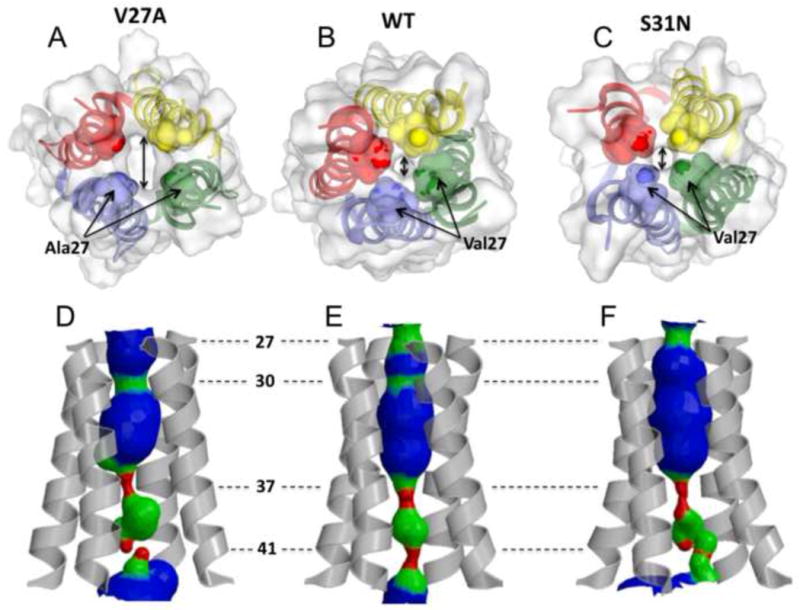
The size of the channel N-terminal entrance of a. the V27A mutant (2KWX), b. WT (2RLF), and c. the S31N mutant (2KIH) indicated by double headed arrow. The side chains at residue position 27 constrict the channel entrance. Mutating valine at this position to alanine doubles the diameter of the channel opening (∼2.5 Å in WT and S31N mutant, ∼5 Å in the V27A mutant). d,e,f The pore surfaces calculated using the program HOLE. d. The WT structure displays two constrictions in the N terminus at the positions 27 and 30. e. The V27A mutant displays one constriction at position 30. f. The S31N mutant is constricted at the position 27, but due to the serine to asparagine substitution at the position 31 the channel forms looser tetramer that result in somewhat larger diameter around Ser30. All of the structures have their C-termini tightly constricted to ∼1.5 Å by side chains of His37 and Trp41.
