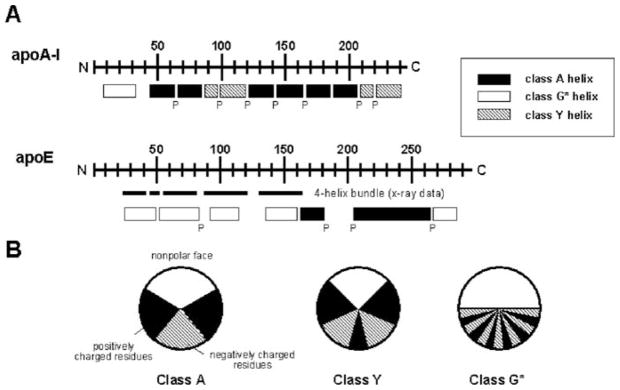
Fig. 7.1.
A Distribution of amphipathic α-helices in the human exchangeable apolipoproteins, apoA-I and apoE. The letter P below the rectangles indicates positions of all proline residues. B Amphipathic helix classes found in the exchangeable apolipoproteins. Classification is based on the distribution of charged residues (see Section 2.1). These figures were adapted from Segrest et al. (1992)