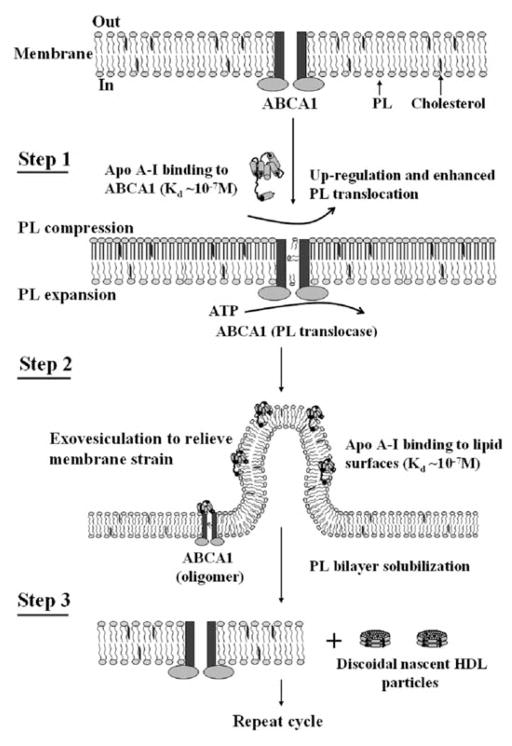
Fig. 7.8.
Mechanism of interaction of apoA-I with ABCA1 and efflux of cellular phospholipids and cholesterol. The reaction in which apoA-I binds to ABCA1 and membrane lipids to create discoidal nascent HDL particles contains three steps. Step 1 involves the high affinity binding of a small amount of apoA-I to ABCA1 located in the plasma membrane PL bilayer; this regulatory pool of apoA-I up-regulates ABCA1 activity, thereby enhancing the active translocation of membrane PL from the cytoplasmic to exofacial leaflet. This translocase activity leads to lateral compression of the PL molecules in the exofacial leaflet and expansion of those in the cytoplasmic leaflet. Step 2 involves the bending of the membrane to relieve the strain induced by the unequal molecular packing density across the membrane and the formation of an exovesiculated domain to which apoA-I can bind with high affinity. This interaction with the highly curved membrane surface involves apoA-I/membrane lipid interactions and creates a relatively large pool of bound apoA-I. Step 3 involves the spontaneous solubilization by the bound apoA-I of membrane PL and cholesterol in the exovesiculated domains to create discoidal HDL particles containing two, three or four apoA-I molecules/particle. In the diagram, the two transmembrane six-helix domains of ABCA1 are represented as rectangles, whereas the two ATPase domains are shown as ovals. The space between the two rectangles represents the chamber in which translocation of PL molecules occurs. Reproduced with permission from Vedhachalam et al. (2007a)