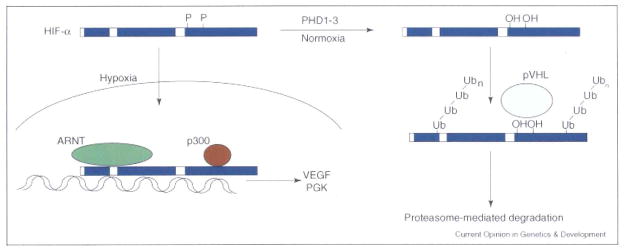
Figure 2.
Regulation of HIF-α stability: continuously transcribed and translated, the HIF-α subunits are degraded under normoxic conditions. Two prolines in the ODD are hydroxylated by PHD1, 2 or 3, allowing recognition by an E3 ubiquitin ligase complex including the VHL tumor suppressor protein. Following pVHL-mediated ubiquitylation, the HIF-α subunits are degraded in a proteasome-dependent fashion. When oxygen levels fall below ~5%, the PHDs are no longer active and the HIF-α subunits can translocate to the nucleus, where they bind co-factors including ARNT and p300 and transactivate hypoxia response genes, such as VEGF and PGK.