Abstract
Ayurvedic dosage forms are very exclusive in its pharmaceutics and therapeutics. Sneha Kalpana is a group of products of medicated taila and ghee, these drugs are treating very wide range of diseases among patients of all age groups. Liposomal system of drug delivery is a new invention in conventional system of medicine. This system is also covering a high degree of objective of therapeutics at different targets successfully. Probably, here is very distinctive similarity between these two on account of their aqueous and oleaginous origin. Most likely, these are two faces of same coin. A brief survey of literature is done here to explore possibilities of further investigation in benefit of mankind by applying wisdom of both fields together. In fact, this is a review paper based on certain hypothesis which may be established or rejected factually by further researches.
Keywords: Aqueous, liposomes, oleaginous, Sneha Kalpana, therapeutics
Introduction
Sneha Kalpana/paka may be defined as “A pharmaceutical process to prepare oleaginous medicaments from the substances like Kalka (herbal paste of different parts of botanicals), Kwatha (specifically prepared decoction in accordance of Ayurvedic principles) or Drava Dravya (any other liquid such as milk, self expressed juices, meat juice, etc.) taken in specific proportion and by subjecting them to unique heating pattern and duration to fulfill certain pharmaceutical parameters, according to the need of therapeutics.”[1]
Means, Sneha Kalpana/paka is a unique dosage form in Ayurveda. Aim of this arrangement is mass transfer of the aqueous and lipid-soluble active principles of all treated herbal drugs and material of animal and mineral origin, if any, in accordance of established formulae quoted in authoritative text books of Ayurveda which should serve therapeutic objectives as per indications of the classical treatise of Ayurveda.[2]
On other hand, in the field of conventional pharmaceutics, various new dosage forms are evolved continuously with basic purposes to increase bioavailability of the drug which may show maximum therapeutic effect. Liposome is one such advanced dosage form in which nanoparticles comprising lipid bilayer membranes surrounding an aqueous interior are formed. The amphiphilic molecules used for the preparation of these compounds have similarities with biological membranes and have been used for improving the efficacy and safety of different drugs. In this dosage form, the active compound can be located either in the aqueous spaces, if it is water-soluble, or in lipid membrane, if it is lipid soluble.[3]
In case of liposomal drug delivery system, tremendous amount of work has been done to formulate these drugs in sustained and controlled release dosage forms for oral and parenteral administration. This is to pursue optimal drug action; functional molecules could be transported by a carrier to the site of action and released to perform their task, for which the carrier itself should be nontoxic, biodegradable, and of suitable shape and size to accommodate wide variety of substances and liposomes that are fulfilling all these parameters.[4] Therefore, liposomes have been widely evaluated for controlled and targeted drug delivery for treatment of cancer, viral infections, and other microbial diseases. Liposomes are found to be suitable for localization of topically applied drugs at or near the site of application, due to the fact that they may act as slow releasing vehicles.[4]
It seems that these two dosage forms, i.e., Sneha Kalpana/Paka of Ayurveda and Liposome of conventional medicine, are very much similar in their origin and character as both are lipoidal in nature.
In the preparation of Sneha paka, particular matter and media in specific ratio is taken and heated along with oil/ghee at a very specific temperature with certain duration till the completion test. Here, the principle is to transfer active constituent of herbs in lipid and water according to its solubility.[5]
Liposomes are prepared on the same pharmaceutical principle; however, in case of liposomes, heating is not only compulsory method of preparation (as the case with products of Sneha paka), and here other methods such as sonication, homogenization, shaking, etc., are also applied. The lipid-soluble compound remains in the outer lipid bilayer and water-soluble component remains in the middle aqueous space.
By keeping above facts in mind, we may assume that Sneha paka may have the same structure and functions as that of liposome (basic hypothesis of this paper), or in other words, liposomes are modified/developed form of the traditional Sneha Kalpana/paka. This concept is being discussed for its similarity and differences, if any, on factual parameters of both sciences, i.e., Ayurvedic pharmacy and modern pharmacy.
Sneha Kalpana/Paka in brief
Almost every classics of Ayurveda define very systematically about manufacturing processes of medicated taila and ghrita (oil/ghee), worth to mention here are Charaka Samhita, Sushruta Samhita, and Ashtanga Hridaya. However, Sharangdhar Samhita is considered as best book for pharmaceutical details of different herbal dosage forms. Therefore, we are putting down some salient preparatory indications of Sneha Kalpana from Sharangdhar Samhita for optimum understanding of concept.
According to Sharangdhar Samhita, Sneha Kalpana may be defined as “the medicament prepared by using one part of Kalka dravya (paste of indicated herbal ingredients), four parts of oil/ghee (commonly sesame oil/cow ghee) and sixteen parts of Drava dravya (liquid media mostly kwatha - decoction of herbs).”[6] Drava dravya may be other than kwath such as jala (water), swarasa (self-expressed herbal juice), kanji (fermented herbal beverage), mansa rasa (meat juice), gomutra (cow urine), etc.
Preparation of Kwatha
For the preparation of kwatha[6] according to hardness of Kwathya dravya (chopped herbs), water should be added for mridu dravya (herbs of soft texture) four times, Madhyama dravya and Kathina dravya (herbs of harder texture) eight times, and for Atyanta kathina dravya (most hard herbs)sixteen times. Following rules are mentioned in the classics[6]:
Rules for preparation of Sneha
If the Drava dravya during preparation of sneha is jala, kwatha, and swarasa, then amount of kalka used should be one-fourth, one-sixth, and one-eighth of sneha, respectively.
When indication of sneha preparation is in dugdha (milk), dadhi (curd), takra (butter milk), and mans rasa (meat juice), the kalka to be used should be one-eighth and water should be added four times for samyaka paka (moderate heating) and complete transfer of active principles.
When Drava dravya are more than five, then each dravya should be taken in the same quantity as that of sneha. If less than five, total quantity of all the liquids should be four times.
When paka (pharmaceutical process for preparation of medicated oil/ghee in which individualized heating process is adopted as per ingredient of formulation) is mentioned by only Kalka dravyas, then water should be added four times of sneha to replace the drava. When paka mentioned by only kwatha dravya, then kalka should be prepared by drugs of kwatha and should be used.
When flower is used as Kalka dravya, then its quantity should be one-eighth to that of sneha.
Sneha siddhi lakshanas, types of Sneha Paka, duration for Sneha paka
In Sharangdhar Samhita, completion tests for medicated oil/ghee, types of Sneha paka viz. Mridu, Madhya, and Khar paka, and duration of manufacturing process as per variation in type and proportion of constituent material are discussed elaborately, which may be applied during preparation of these Sneha kalpa. These parameters of completion tests and other measures may also be used as distinguish criteria for quality control of products.[6]
Therapeutic multiplicity of Sneha Kalpana/Paka
Sneha kalpa, that is, medicated oils/ghee of Ayurvedic dosage forms, are used in therapeutics both topically and systemically. Thus, we can see a wide variety of uses of Sneha Kalpana, some of which are under mentioned.[7]
Nasya Kalpana (e.g., Shadabindu Taila, Anu Taila)
Mukha Kalpana (e.g., Irimedadi Taila)-Two types-Gandusha and Kawala
Netra Kalpana-(e.g., Triphala Ghrita)
Abhyanga-(e.g., Dashamula Taila)
Anuvasana Basti-(e.g., Saindhavadi Anuvasana Taila)
Uttarbasti, Pichu-(e.g., Mushakadya Taila)
Snehana in Panchakarma therapy-(e.g., Pancha Prasritiki Peya)
Internal administration-(e.g., Panchatikta Ghrita, Kshira Bala Taila) for shodhana/nourishment
In nonhealing ulcer-(e.g., Jatyadi Ghrita)
Basics of Liposomal Theory
Etymology
The name liposome is derived from two Greek words: “Lipos” meaning fat and “Soma” meaning body. Liposomes can be formed in variety of sizes as unilamellar or multilamellar construction, and its name relates to its structural building blocks, phospholipids, and not to its size.[8]
Discovery
Liposomes were first described by British hematologist Dr. Alec D Bangham FRS in 1961 (published 1964) at the Babraham Institute, in Cambridge.[8]
Structure
In nature, phospholipids are found in stable membranes composed of two layers (a bilayer). In the presence of water, the heads are attracted to water and line up to form a surface facing the water. The tails are repelled by water and line up to form a surface away from the water. In a cell, one layer of heads faces outside of the cell, attracted to the water in the environment and another layer of heads face inside the cell, attracted by the water inside the cell. The hydrocarbon tails of one layer face the hydrocarbon tails of the other layer, and the combined structure forms a bilayer.[8]
Drug delivery by liposome
The size of these spheres is very small, in the order of a nanometer. The spheres are hollow inside and enclose some of the liquid material in which they were formed (inclusion). Because of the small size of the phospholipids molecule and microspheres, they can pass through the epidermis and act as a carrier for the enclosed substances. It is postulated that when they reach the outside of a living cell membrane in the dermis, they may become accepted as part of the membrane, being of the same composition. Thus, they are able to carry with them any enclosed substances into the dermis and to the individual cells.[9]
Application of liposome
Liposome is used for drug delivery due to their unique properties. A liposome is capable to deliver hydrophilic and lipophilic substances which encapsulate a region on aqueous solution inside a hydrophobic membrane; dissolved hydrophilic solutes cannot readily pass through the lipids. Hydrophobic chemicals can be dissolved into the membrane, and in this way, liposome can carry both hydrophobic molecules and hydrophilic molecules. To deliver the molecules to sites of action, the lipid bilayer can fuse with other bilayers such as the cell membrane, thus delivering the liposome contents.[8]
By making liposome in a solution of drugs (which would normally be unable to diffuse through the membrane, i.e., hydrophilic drugs), they can be (indiscriminately) delivered past the lipid bilayer. There are three types of liposomes—MLV (multilamellar vesicles), SUV (Small Unilamellar Vesicles), and LUV (Large Unilamellar Vesicles). These are used to deliver different types of drugs.[8]
As inference, we may understand that liposomes are concentric bilayered structures made of amphipathic (may transport both ways, i.e., inside out and outside in) phospholipids and depending on the number of bilayer. Liposomes are classified as MLV, SUVs, or LUVs. They range in size from 0.025 to 10 μ in diameter. The size and morphology of liposomes are regulated by the method of preparation and composition. Liposomes are used for delivery of drugs, vaccines, and genes for a variety of disorders.[10]
Discussion
Applicability of medicated oil and ghee of Ayurveda
Ayurveda, a holistic healthcare system, prescribes usage of different medicated taila and ghrita for applications on the body, with or without massage for providing health benefits and to treat specific indications. Although most of the medicated oils are for external usage, certain types of medicated oils are administered orally also.[11] However, medicated ghrita of Ayurveda are used systemically orally.
Mahanarayan Taila, Pancha Guna Taila, Maha Vishagarbha Taila, Shadbindu Taila, and Maha Marichyadi Taila are few examples from among medicated oils of Ayurveda which are indicated for particular disease. Brahmi Ghrita, Jatyadi Ghrita, and Maha Triphaladi Ghrita are another group of products of Sneha Kalpana which are prescribed by Ayurvedic physicians to treat certain disorders of different system of human body.
Apart from these, varieties of Ayurvedic Taila/Ghee are used in different stages of Pancha Karma (five specific procedure, viz. 1. Vamana—therapeutic emesis, 2. Virechana—therapeutic purgation, 3. Niruha Basti—cleansing enema, 4. Anuvasana Basti—retention enema, 5. Nasya—therapeutic errhine) therapy of Ayurveda. If one tries to analyze the different route of administration (topical and systemic) of these medicated oil and ghrita and level of therapeutic effectiveness of the same, it may be clearly established that these all reached to the target (site of therapeutic action) through physiological mechanism and serve the purpose.
Many pharmaceutical studies are supporting the facts that there are very crucial changes taking place in Siddha Sneha (medicated oil/ghee) during the pharmaceutical process which may be responsible for many pharmaceutical and therapeutic properties of medicated oil/ghee.[12,13]
But, unfortunately, we have not studied till date about pharmacokinetic and pharmacodynamic of these drugs on parameters of contemporary science. However, on other hand, this is also true that many Ayurvedic physicians have tremendous experience of activity of all these drugs and have understanding of action of all these on the basis of Ayurvedic doctrines.
Applicability of liposomal drug delivery system
Liposomes have been widely used as drug carrier in topical treatment of diseases, especially in dermatology. They are capable to incorporate a variety of hydrophilic and hydrophobic drugs, to enhance the accumulation of drug at the administration site, and to reduce side effects. Liposomes can provide sustained and/or controlled release of entrapped drug. Liposomal system allows for a high accumulation of drug in the skin, with relatively low permeation flux as compared with the conventional dosage form.[14]
To substantiate our understanding of these lipoidal drugs (oleaginous formulation of Ayurveda), we are referring here some studies of drugs of liposomal nature of conventional system of medicine which prove our belief that Ayurvedic drugs of Sneha Kalpana are working systemically.
As topical drugs
Liposome
Liposomal encapsulation showed more drug retention compared with plain drug gel and plain drug cream. The higher drug skin retention in case of liposomal gel may be due to creation of reservoir effect for drug in skin due to deposition of other components of liposomes with drug into the skin, thereby increasing the drug retention capacity into the skin.[14]
In a study, the liposomal product of ketoconazole was prepared with the view to improve therapeutic response and reduce the possible adverse symptoms. Here, liposomes of ketoconazole were prepared using thin-film hydration technique. Percentage entrapment efficiency was optimized after studying the effect of various process and formulation variables. And, the liposomal delivery of ketoconazole was found better.[4]
In another study, the in vitro permeation of ketoconazole using Wistar albino rat skin from liposomal gel was compared with that of plain drug gel and also with plain drug cream containing 2% w/w of ketoconazole. The release of ketoconazole from liposomal gel was much slower than from non-liposomal formulations. Gel containing liposomal ketoconazole showed maximum antifungal activity after 30 hours over plain ketoconazole gel and cream formulations. This proved control drug delivery phenomenon of liposomal theory.[14]
Sneha Kalpana
Above referred studies, if correlated with Ayurvedic taila and ghrita which are used topically, may answer many queries related to its absorption and delivery, provided a study should be planned taking samples of Ayurvedic medicaments.
As nanomedicine
Liposomes
Recently, increasing attention has been focused on Solid Lipid Nanoparticles (SLN), because it offers advantages viz. possibility of controlled drug release and drug targeting, increased drug stability, high drug payload, no biotoxicity of the carriers, avoidance of organic solvents, no problems with respect to large scale production, cost effective, and sterilization. Furthermore, one study established that the incorporation of hydrophilic lamivudine in SLN carriers results in decreased bone marrow toxicity, increased bioavailability, and enhanced antiviral activity.[15] This study claimed that lamivudine in SLN present cross blood brain barrier and act on target site.
As per one research work, the cumulative percentage drug release of nimesulide was found to be approximately 60% in 24 hours and release behavior was in accordance with Higuchi-equation. The results indicate that the SLNs are a promising controlled-release system. It may also allow a reduction in dosage and a decrease in systemic toxicity.[16]
In nut shell, one may suggest that during the past 30 years, liposomes have received increased attention from the scientific community, as well as from the industry, due to the possibility of being a pharmaceutical carrier for numerous problematic drugs. This success seems to drive the present interest on the field with more than 2 000 papers and reviews per year. We will concentrate in two aspects of the utilization of Liposomes: As carriers either for macromolecules, for the treatment of cancer and inflammatory disorders, or for small molecular weight drugs, for the treatment of intracellular infectious diseases.[17]
Sneha Kalpana
Taking analogy from same, we propose that Shadabindu Taila indicated for Pratishyaya (rhinitis and allergic disorders) and Brahmi Ghrita indicated for the Unmad and Apasmar (Mental disorders) may be crossing blood brain barrier and showing their therapeutic effects due to its lipoidal structure.[18] Few research works should be planned to validate the claim of Ayurveda as per scientific language of today.
This is possible here that Panch Guna Taila, which is considered as pain relieving drug in Vatic (group of diseases in which pain is a prominent symptom) disorders and many more, are providing relief on same mode of action.
As nutraceuticals
Liposome
Nutraceuticals are food components that have health benefits beyond traditional nutritional value. Novel biotechnology tools, like immobilization, have also been applied for the isolation and incorporation of such food components in ordinary foods. Successful synthesis of nutraceuticals has been reported by employing immobilized lipases (part of liposome), such as those from C. antarctica and L. ruteri. Lipases are also used in the synthesis of the artificial sweetener sucralose by regioselective hydrolysis of octaacetylsucrose.[19]
Sneha Kalpana
Study may be planned to see the availability of lipase during manufacturing process of taila and ghrita. In Ayurveda, too many ghrita and taila are used for nutraceutical purpose, such as Kushmand Ghrita, Amrita prash Ghrita, and Ashwagandha Ghrita.[20]
Summary of correlation
Our overall submission is that liposomes are lyotropic (a material which forms liquid crystal phases because of addition of solvent. These are amphiphilic molecules comprised of hydrophobic and hydrophilic both groups) liquid crystals composed of relatively biocompatible and biodegradable materials and consist of an aqueous core entrapped by one or more bilayers of natural and/or synthetic lipids. Drugs with widely varying lipophilicities can be encapsulated in liposomes either in the phospholipid bilayer, in the entrapped aqueous core, or at the bilayer interface. Reformulation of drugs in liposomes has provided an opportunity to enhance the therapeutic indices of various agents, mainly through the alteration of biodistribution. They are versatile drug carriers, which can be used to control retention of entrapped drugs in the presence of biological fluids, control vesicle residence in the systemic circulation or other compartments in the body, and enhance vesicle uptake by target cells.[21]
Most attracting about liposomes are that these are composed of natural lipids and are biodegradable, biologically inert, weakly immunogenic,[22] produce no antigenic or pyrogenic reactions, and possess limited intrinsic toxicity.[23] Therefore, drugs encapsulated in liposomes are expected to be transported without rapid degradation and minimum side effects to the recipients. Moreover, efforts have been made to assess the specificity of drug carriers to the target organs, cells, or compartments within the cells.[24] Liposomes are better suited for assessing their targetable properties because of the ease of modifying their surface when compared with other drug carriers such as nanoparticles[25,26] and microemulsions[27] Many approaches have been attempted to achieve targetable properties, including noncovalent association of cell-specific antibodies with liposomes.
The different variants in proportion of Kalka, Kwath, and Sneha, as quoted in description with reference of Sharangdhar Samhita, may have its own rationality in terms of pharmaceutics and therapeutics. We found some research papers which are showing change in therapeutic efficacy when proportions of components of liposome preparation are changed.
This has been established that in conventional pharmacy, for a said synthetic molecule, liposomal drug form is more potent in comparison of other dosage form. Here by, we quote an excellent study which was undertaken to investigate the impact of formulation factors and adjuvants on the expression of biological activity of Tinospora cordifolia (Willd) Miers in two dosage form, i.e., swarasa (fresh juice) and medicated ghrita of same. In this study, the adaptogenic effect of three samples of Guduchi Ghrita, prepared using plain ghee (clarified butter) obtained from three different sources, was studied in albino rats and compared with expressed juice of stem of Guduchi. The test preparations were evaluated against forced swimming-induced hypothermia, gastric ulceration, and changes in the hematological parameters. The test drug given in the form of “ghrita” produced better effect in comparison with the expressed juice.[28]
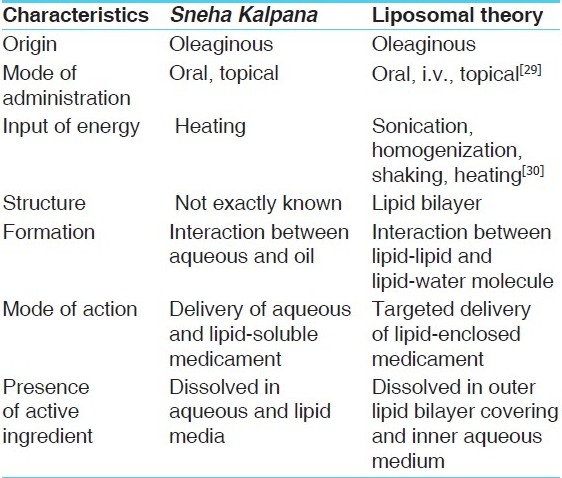
Thus, we are of opinion that here is very momentous connection between products of Sneha Kalpana and liposomes of Ayurveda and Allopathy, respectively, which are abridge in Table 1.
Table 1.
Similarities between Sneha Kalpana and liposomes
Conclusion
An elegant literary appraisal based on facts derived from 2000-year-old medical system to recent researches are indicating the unique methodology of general, sustain, and control pattern of drug delivery at target which is possible with dosage forms of Sneha Kalpana and liposomal drug delivery. With very genuineness, these are serving a range of therapeutic objectives with inimitable approach on virtue of its unique structural specifications.
We request all laboratory researchers to please validate this hypothesis of correlation which may provide us a new height in application of Sneha Kalpana, if technological development of liposomal drug delivery system is adapted to products of Sneha Kalpana in their pharmaceutics and therapeutics.
Conflict of Interest
The objective of this review write up is to ascertain a bridge between traditional wisdom and current trend of drug delivery system where progression of new pioneering invention may be utilized to fortify traditional dosage form, and newer intrinsic worth may be added to concurrent dosage form taking lead from ancient knowledge.
We do hope that there will be no conflict of interest for anyone with this theme at present. Both authors have no vested interest attached to this review.
Acknowledgments
We acknowledge contribution of Prof. S. S. Savirkar, former Vice Chancellor, Gujarat Ayurved University, Jamnagar, for providing conceptual lead for this correlation.
References
- 1.Onten CS, Kumar Vikas Chaudhary A. Varanasi: BHU; 2009. Study of Stability (Saveeryata Avadhi) of Samanya and Panchavartita Panchtikta Ghrita, (M.D.Ayu. dissertation) [Google Scholar]
- 2.Dhruve K, Chaudhary A. Sneha kalpana- A probable pharmaceutical explanation. Aryvaidyan. 2007;20:181–9. [Google Scholar]
- 3.Medina C, Santos M, Radomski A, Corrigan V, Radomski MW. Nanoparticles: Pharmacological and toxicological significance. Br J Pharmacol. 2007;150:552–8. doi: 10.1038/sj.bjp.0707130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Patel RP, Patel H, Baria A. Formulation and evaluation of Liposomes of Ketoconzole. Int J Drug Deliv Technol. 2009;1:16–23. [Google Scholar]
- 5.Aarathi TS, Chaudhary A. Gujarat, India: Gujarat Ayurveda University; 2005. Pharmaceutical standardization of Ksheerbala Taila – Shelf life study, M Pharma Ay.dissertation. [Google Scholar]
- 6.Tripathi B, Sharangdhar S, Dipika Hindi Vyakhya. Varanasi: Chaukhamba Surbharati Prakashan; 2004. Madhyama Khanda 9/1, 9/3-4, 9/8-11, 9/12-13, 9/14-15, 9/18. [Google Scholar]
- 7.Sharma PV. Sushruta Chikitsa 31/3. 1st ed. Vol. 7. Varanasi: Oriental Publisher and Distributors; 2000. Sushruta Samhita with English translation of text and Dalhan's commentary along with critical notes. Chaukhamba Vishva Bharati. [Google Scholar]
- 8.Liposome from Wikipedia, the free encyclopedia. [Last accessed on 2010 Apr 03]. Available from: http://en.wikipedia.org/wiki/Liposome .
- 9.Adair's D Cosmetics, Liposomes. [Last accessed on 2010 Apr 03]. Available from: Http.//www.dadair.com/liposomes.htm .
- 10.Kshirsagar NA. Drug Delivery Systems. Indian J Pharmacol. 2000;32:S54–61. [Google Scholar]
- 11.Loharkar P, Ramitha K, Bansal V, Anant Narayanan DB. A comparative Evaluation of Medicated Oils----Processes. Indian J Pharm Sci. 2009;71:656–62. doi: 10.4103/0250-474X.59548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saxena RB, Alam M. Standardisation of Murchhita katu taila. J Resn Ayurveda Siddha. 1988;3-4:204–208. [Google Scholar]
- 13.Rao NV, Rao Shankar K, Dixit SK. Standardisation of Ksheerbala taila. Anc Sci Life. 1996;16:21–5. [PMC free article] [PubMed] [Google Scholar]
- 14.Patel RP, Patel H, Baria A. (Part II) Formulation and evaluation. Int J Drug Deliv Technol. 2009;1:42–5. [Google Scholar]
- 15.Patil P, Sharma A, Dadarwal S, Sharma V. Development of Solid lipid Nanoparticles of Lamivudine. Int J Drug Deliv Technol. 2009;1:136–8. [Google Scholar]
- 16.Jain P, Mishra A, Yadav SK, Patil UK, Baghel US. Formulation Development and Characterization. Int J Drug Deliv Technol. 2009;1:124–7. [Google Scholar]
- 17.Cruz EM, Gasper MM, Curovo LM, Caravalerhio M, Martin BM. Application of Liposome in Drug delievery, Unit of “Novas Formas de Agentes Bioactivos”. Dept. Biotechnology/INETI, Estrada do Paço do Lumiar 22, 1649.038, Lisboa, Portugal. 2005 Achliya GS, Wadokar S G, Dorle A K . Evaluation of CNS activity of Brahmi Ghrita. Indian J Pharmacol 2005;37:33-36. [Google Scholar]
- 18.Gupta KA. 14th ed. Varanasi: Chaukhamba Sanskrit Sansthan; 2003. Commentary on Astang Hridaya, Vagbhatta. [Google Scholar]
- 19.Aravindan R, Anbumathi P, Viruthagiri T. Lipase application in food industry. Indian J Biotechnol. 2007;6:141–58. [Google Scholar]
- 20.Goel P, Goel K, Vijya Kumar SG, Singh A, Katare OP, Mishra DN. Liposomal drug delivery systems – Clinical applications. Acta Pharm. 2005;55:1–25. [PubMed] [Google Scholar]
- 21.Rooijen N, Nieuwmegen R. Liposomes in immunology: Multilamellar phosphatidylcholine liposome as a simple biodegradable and harmless adjuvant without any immunogenic activity of its own. Immunol Commun. 1980;9:243–56. doi: 10.3109/08820138009065997. [DOI] [PubMed] [Google Scholar]
- 22.Campbell PI. Toxicity of some charged lipids used in liposome preparation. Cytobios. 1983;37:21–6. [PubMed] [Google Scholar]
- 23.Gregoriadis G. Targeting of drug. Nature. 1977;265:407–11. doi: 10.1038/265407a0. [DOI] [PubMed] [Google Scholar]
- 24.Grislain L, Couvreur P, Lenaerts V, Roland M, Deprez-Decampeneere Spieser P. Pharmakokinetics and distribution of a biodegradable drug-carrier. Int J Pharmacol. 1983;15:335–8. [Google Scholar]
- 25.Illum L, Jones PD, Kreuter, Baldwin RW, Davis SS. Adsorption of monoclonal antibodies to polyhexylcyanoacrylate nanoparticles and subsequent immunospecific binding to tumor cells. Int J Pharm. 1983;17:65–9. [Google Scholar]
- 26.Hashida M, Takashi Y, Muranishi S, Sezaki H. An application of water in oil and gelatin microsphere in oil emulsions to specific delivery of anticancer agents into stomach lymphatics. J Pharmacokin Biopharmacol. 1977;5:241–4. doi: 10.1007/BF01065398. [DOI] [PubMed] [Google Scholar]
- 27.Mizushima Y, Hamano T, Yokoyama K. Use of a lipid emulsion as a novel carrier for corticosteroids. J Pharm Pharmacol. 1982;34:49–53. doi: 10.1111/j.2042-7158.1982.tb04678.x. [DOI] [PubMed] [Google Scholar]
- 28.Savrikar SS, Dole V, Ravishankar B, Shukla V. A comparative pharmacological investigation of three samples of ‘Guduchi ghrita’ for adaptogenic activity against forced swimming induced gastric ulceration and hematological changes in albino rats. Int J Ayurveda Res. 2010;1:67–72. doi: 10.4103/0974-7788.64399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gregoriadis G. Interactions of liposomes with the biological milieu. [Last accessed on 2010 Jul 15]. p. 104. Available from: http://books.google.co.in .
- 30.Reza Mozafari M. Vol. 10. Palmerston North, New Zealand: Massey University; 2005. Cellular and Molecular Biology Letters. Liposome..An overview of manufacturing techniques, Riddet Centre. Massey University, Private Bag 11222; pp. 711–9. [PubMed] [Google Scholar]


