Abstract
An ampC gene was cloned from a clinical isolate of Acinetobacter baumannii (strain RAN). DNA sequencing and primer extension studies showed that ampC is transcribed from a promoter contained within a putative insertion sequence element which has been found to abut several different genes in Acinetobacter spp.
Acinetobacter baumannii has emerged as a significant nosocomial pathogen, especially in intensive care units (4). The treatment of infections due to this organism can present therapeutic problems, primarily because A. baumannii is often resistant to multiple antibiotics, including the β-lactam antibiotics (11, 12). A number of chromosomal β-lactamases have been described in A. baumannii (2, 13); however, only the chromosomal cephalosporinase gene, ampC, has been cloned from this organism (5). As the transcription site of ampC was not established, the promoter for this gene is unknown (5). This communication describes the transcription and genetic environment of ampC from a clinical isolate of A. baumannii (strain RAN).
Strain RAN was isolated from blood and identified in the microbiology laboratory of Groote Schuur Hospital, Cape Town, South Africa. MICs of β-lactams with Etest strips (AB Biodisk, Solna, Sweden) for strain RAN are shown in Table 1. Similar determinations showed that strain RAN was resistant to amoxicillin/clavulanate (MIC, >256 μg/ml) and piperacillin/tazobactam (MIC, >256 μg/ml). The MIC of cephalothin (Sigma) was determined with a twofold agar dilution method on IsoSensitest agar (Oxoid, Basingstoke, United Kingdom) and an inoculum of 104 CFU/spot.
TABLE 1.
β-Lactam MICs for A. baumannii strain RAN and comparison of MICs for E. coli(pMERL100) and E. coli(pGER1)a
| Antibiotic | MIC (μg/ml)
|
||||
|---|---|---|---|---|---|
| A. baumannii RAN | E. coli JM109 (pMERL100) | E. coli JM109 | E. coli TG1 (pGER1) | E. coli TG1 | |
| Cefuroxime | >256 | 32 | 2 | >256 | 4 |
| Cefoxitin | >256 | 24 | 3 | 4 | 4 |
| Cefotaxime | >32 | 0.25 | 0.025 | 4 | ≤0.125 |
| Ceftazidime | 16 | 0.75 | 0.25 | 16 | ≤0.125 |
| Cephalothin | >128 | >128 | NDb | ND | ND |
Genomic DNA from A. baumannii strain RAN was extracted (3), digested with HindIII, and ligated to similarly digested pBGS8 (17). Recombinant plasmids were introduced into Escherichia coli JM109 (18), which were made competent for DNA uptake with the CaCl2 shock procedure (7). Recombinant plasmid DNA was extracted (15), and one plasmid, designated pMERL100, containing a HindIII insert of approximately 5.0 kb conferred resistance to cephalothin and cefuroxime and diminished susceptibility to cefotaxime and cefoxitin on its E. coli host (Table 1). This resistance phenotype is different from that of E. coli containing the A. baumannii ampC gene in a similar vector (pBGS18) designated pGER1 (5) (Table 1). The cefoxitin MIC (24 μg/ml) for E. coli(pMERL100) (Table 1) suggested activity against this antibiotic. However, with a spectrophotometric assay, hydrolysis of this antibiotic was not demonstrated for β-lactamase extracts of E. coli(pMERL100) (data not shown).
A portion (3.123 kb) of the 5.0-kb insert was sequenced on both strands (accession no. AY325306). The deduced amino acid sequence of the ampC structural gene showed 98.4, 98.7, 98.7, and 99% identity to ampC from A. baumannii RYC52763/97 (5), A. baumannii ABAC1, A. baumannii ABAC2 (14), and Oligella urethralis COH-1 (14), respectively. Sequencing data showed that the 5′ end of the structural ampC gene is adjacent to a sequence, called the homologous sequence, which contains features of insertion sequence elements, including outwardly directed promoters, found at the 5′ ends of a number of genes from Acinetobacter spp. (16). The ampC start codon is 9 bp downstream from the nucleotide (G) which defines the boundary of the homologous sequence (16).
The precise initiation site of the ampC transcript was determined by primer extension analysis. Total RNA was extracted from exponential-phase cultures of A. baumannii strain RAN, containing cefoxitin (30 μg/ml), with hot acidic phenol (9). A primer (5′-TACCTGGCACATCATATT-3′) that annealed to sequences downstream of the ATG initiation codon of the ampC gene was synthesized and labeled with indodicarbocyanine in the Department of Molecular and Cell Biology, University of Cape Town, Cape Town, South Africa. The primer was annealed to the mRNA template and extended with Moloney murine leukemia virus reverse transcriptase (Promega, Madison, Wis.) and an equimolar deoxynucleoside triphosphate mixture. The primer extension products were precipitated, reconstituted in ALF stop buffer (Pharmacia Biotech), heated, and analyzed in conjunction with sequencing reaction products performed on the corresponding DNA. Analysis of the products was performed with the ALFexpress automated DNA sequencer (Amersham Biosciences) in the Department of Molecular and Cell Biology, University of Cape Town.
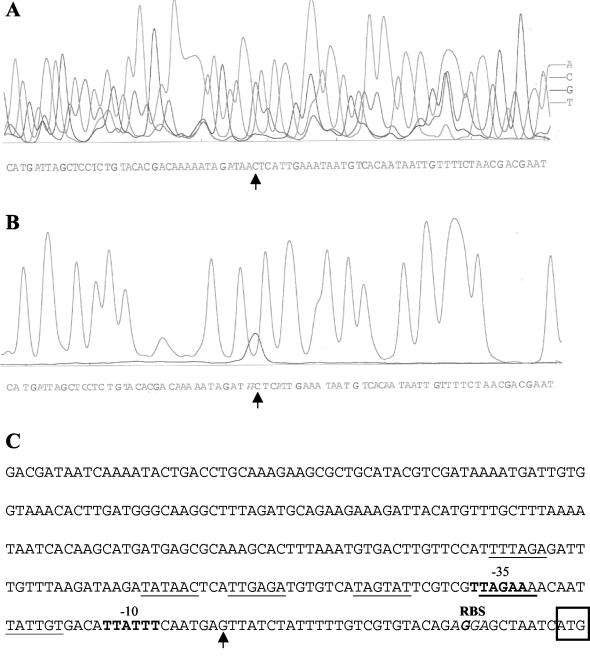
The primer extension product was mapped to a G located in the putative insertion sequence, 35 nucleotides upstream of the ampC start codon (Fig. 1). The hexamers TTAGAA (−35) and TTATTT (−10), separated by 16 bp, upstream of the transcription start site show similarity to promoter consensus sequences recognized by Eσ70. By and large, there is good correlation between promoter strength and the degree of similarity to the Eσ70 consensus hexamers TTGACA (−35) TATAAT (−10) and to the consensus length (17 bp) separating them (8). In terms of these parameters, the ampC promoter is weak. Different, properly aligned promoters (Fig. 1) are present in the putative insertion sequence upstream of ampC; thus, although only a single primer extension product was obtained, it is possible that transcription initiation could proceed from more than one promoter, resulting in an increase in AmpC production. Interestingly, hyperproduction of AmpC in A. baumannii strains has been reported recently (6). The hyperproducing strains were resistant to ceftazidime, and the ampC genes were located adjacent to the putative insertion sequence element (6), supporting the notion that transcription from different promoters within this element could result in an increase in AmpC.
FIG. 1.
Mapping of transcription initiation site of A. baumannii RAN ampC by primer extension analysis. DNA sequencing fluorograms generated with indodicarbocyanine-labeled primer are shown, with the peaks corresponding to the nucleotides indicated. (A) DNA sequence of template strand upstream of ampC initiation codon, starting at the initiation codon (CAT). (B) T sequencing reaction products and primer extension product. The transcription start site is indicated by the vertical arrow. (C) Nucleotide sequence of the regulatory region (300 bp) upstream of the ampC initiation codon (boxed). TTAGAA (−35)-N16-TTATTT (−10) upstream of the transcription start site are shown in bold. Additional −35 and −10 hexamers are underlined. The coincident G demarcating the start of the homologous region is shown in bold. The ribosome-binding site (RBS) is indicated in italics.
The ampC-type gene blaABA-1 in O. urethralis, suggested to have originated in A. baumannii, was identified downstream of a different insertion sequence element, ISOur1 (14). Putative promoters identified for the ampC in O. urethralis suggest that it is transcribed from a hybrid promoter, the −35 hexamer being located in the insertion sequence element. Subsequently, this sequence was identified in A. baumannii strains (6). Taken together, these data indicate that ampC-type genes are regulated differently in disparate Acinetobacter strains.
As different genes were identified downstream of the homologous region detected in A. baumannii strain PAU (16), we determined whether ampC is located in a different genetic environment in this strain. PCR assays with primers complementary to sequences in the homologous region (5′-ATAGTTTCACCCGACCA-3′) and ampC (P1) (5) showed that in strain PAU, ampC is adjacent to the homologous region (data not shown). Thus, this strain contains at least two copies of the homologous region or putative insertion sequence. A more intensive study is being carried out to determine the prevalence of the putative insertion sequence element, and the identity of the sequences flanking this region, in A. baumannii strains.
The DNA sequence (1,017 bp) at the 3′ end of ampC showed no similarity to the corresponding region of the previously described ampC (5). The sequence contains one open reading frame of 960 bp; the translation product of this open reading frame has 52% identity with the C terminus (amino acids 477 to 792) of ComA from Acinetobacter calcoaceticus BD413. In the naturally competent A. calcoaceticus BD413, ComA has been proposed to be involved with DNA transfer across the cytoplasmic membrane during the period of competence (10). The protein from strain RAN is much shorter (320 amino acids) than its counterpart (793 amino acids) in A. calcoaceticus, suggesting that it is one of the shorter homologues which has been described from a large number of bacterial species (http://tigrblast.tigr.org/web-hmm/accession no. TIGR00361). The function of the shorter homologues is unknown, and for the most part they have been identified in bacteria that are not known to be naturally competent, suggesting that strain RAN is not naturally competent for the uptake of DNA. Whether this applies to all A. baumannii strains remains to be determined.
Acknowledgments
This work was supported by a grant from the University of Cape Town to B.G.E. E.C.N. is the recipient of a Sainsbury Scholarship.
REFERENCES
- 1.Altschul, S. F., T. L. Madden, A. A. Schäffer, J. Zhang, Z. Zhang, W. Miller, and D. J. Lipman. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amyes, S. G. B. and H-K. Young. 1996. Mechanisms of antibiotic resistance in Acinetobacter spp.—genetics of resistance, p. 185-224. In E. Bergogne-Bérézin, M. L. Joly-Guillou, and K. J. Towner (ed.), Acinetobacter, microbiology, epidemiology, infections, management. CRC Press, Boca Raton, Fla.
- 3.Ausubel, F. M., R. Brent, R. E. Kingston, D. D. Moore, J. G. Seideman, and J. A. Smith. 1989. Current protocols in molecular biology. Greene Publishing Associates and Wiley-Interscience, New York, N.Y.
- 4.Bergogne-Bérézin, E., and K. J. Towner. 1996. Acinetobacter spp. as nosocomial pathogens: microbiological, clinical, and epidemiological features. Clin. Microbiol. Rev. 9:148-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bou, G., and J. Martínez-Beltrán. 2000. Cloning, nucleotide sequence, and analysis of gene encoding an AmpC β-lactamase in Acinetobacter baumannii. Antimicrob. Agents Chemother. 44:428-432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Corvec, S., N. Caroff, E. Espaze, C. Giraudeau, H. Drugeon, and A. Reynaud. 2003. AmpC hyperproduction in Acinetobacter baumannii clinical strains. J. Antimicrob. Chemother. 52:629-635. [DOI] [PubMed] [Google Scholar]
- 7.Dagert, M., and S. D. Ehrlich. 1979. Prolonged incubation in calcium chloride improves the competence of Escherichia coli cells. Gene 6:23-28. [DOI] [PubMed] [Google Scholar]
- 8.DeHaseth, P. L., M. L. Zupancic, and M. T. Record. 1998. RNA polymerase-promoter interactions: the comings and goings of RNA polymerase. J. Bacteriol. 180:3019-3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Elisha, B. G., and L. M. Steyn. 1991. Cloning of AAC(3) and AAD(2") genes from Acinetobacter: differential expression in the host strain. Curr. Microbiol. 22:259-263. [Google Scholar]
- 10.Friedrich, A., T. Hartsch, and B. Averhoff. 2001. Natural transformation in mesophilic and thermophilic bacteria: identification and characterization of novel, closely related competence genes in Acinetobacter sp. strain BD413 and Thermus thermophilus HB27. Appl. Environ. Microbiol. 67:3140-3148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Henwood, C. J., T. Gatwood, M. Warner, D. James, M. W. Stockdale, R. P. Spence, K. J. Towner, D. M. Livermore, and N. Woodford. 2002. Antibiotic resistance among clinical isolates of Acinetobacter in the United Kingdom, and in vitro evaluation of tigecycline (GAR-936). J. Antimicrob. Chemother. 49:479-487. [DOI] [PubMed] [Google Scholar]
- 12.Karlowsky, J. A., D. C. Draghi, M. E. Jones, C. Thornsberry, I. R. Friedland, and D. Sahm. 2003. Surveillance for antimicrobial susceptibility among clinical isolates of Pseudomonas aeruginosa and Acinetobacter baumannii from hospitalized patients in the United States, 1998-2001. Antimicrob. Agents Chemother. 47:1681-1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.López-Hernández, S., T. Alarcón, and M. López-Brea. 2001. Biochemical characterization of chromosomal cephalinosporinases belonging to the Acinetobacter baumannii complex. Clin. Microbiol. Infect. 7:218-226. [DOI] [PubMed] [Google Scholar]
- 14.Mammeri, H., L. Poirel, N. Mangeney, and P. Nordman. 2003. Chromosomal integration of cephalinosporinase gene from Acinetobacter baumannii into Oligella urethralis as a source of acquired resistance to β-lactams. Antimicrob. Agents Chemother. 47:1536-1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 16.Segal, H., R. Thomas, and B. G. Elisha. 2003. Characterization of class 1 integron resistance gene cassettes and the identification of a novel insertion sequence-like element in Acinetobacter baumannii. Plasmid 49:169-178. [DOI] [PubMed] [Google Scholar]
- 17.Spratt, B. G., P. J. Hedge, T. S. Heesen, A. Edelman, and J. K. Broome-Smith. 1986. Kanamycin-resistant vectors that are analogues of plasmids pUC8, pUC9, pEMBL8, and pEMBL9. Gene 41:337-442. [DOI] [PubMed] [Google Scholar]
- 18.Yanisch-Perron, C., J. Veira, and J. Messing. 1985. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp 18 and pUC19 vectors. Gene 33:103-119. [DOI] [PubMed] [Google Scholar]