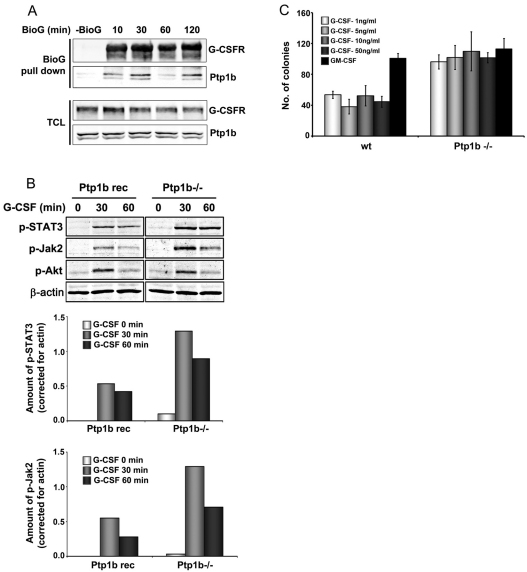
Fig. 5.
Ptp1b interacts with G-CSFR and attenuates signalling. (A) Ptp1b immunoprecipitation from HEK293T cells transfected with lysine-less pBABE-K5R-G-CSFR and pJ3H-Ptp1b-HA constructs. Cells were deprived of growth factor for 4 hours, followed by stimulation with biotinylated G-CSF (BioG) for the indicated times. Precipitates were collected on streptavidin-coated beads. Blots were stained for G-CSFR and Ptp1b; TCL total cell lysate. (B) Western blot analysis of phospho-STAT3, phospho-Jak2 and phospho-Akt in Ptp1b−/− and reconstituted MEFs stably expressing G-CSFR (Ptp1b rec); stimulation conditions as for Fig. 4C. β-actin served as loading control in all experiments. Ptp1b−/− and reconstituted MEFs expressed comparable G-CSFR expression levels, as determined by flow cytometry. Histograms show quantifications of phospho-STAT3 and phospho-JAK2 (Odyssey 3.0). (C) Colony assays of Ptp1b−/− and wild-type littermate control bone marrow cells. Culture conditions were similar to those described for A.