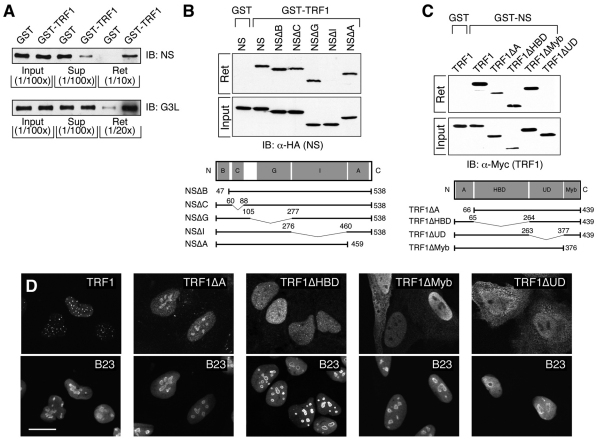
Fig. 1.
NS and GNL3L bind directly to two separate domains of TRF1. (A) Purified NS and GNL3L proteins (40 μg) were incubated with purified Sepharose-bound GST–TRF1 protein (4 μg). Fractions of the input (1/100th), unbound supernatant (Sup, 1/100th), and retained lysates (Ret, 1/10th–1/20th) were immunoblotted (IB) with anti-NS and anti-GNL3L (G3L) antibodies. The pull-down results confirmed that NS and GNL3L bind TRF1 directly. (B) GST pull-down assays showing that the I-domain of NS is required for its TRF1 interaction. (C) Conversely, the UD domain of TRF1 is required for its interaction with NS. Bottom panels in B and C depict the deletion mutants of NS and TRF1. Bent lines and numbers denote the deleted regions and amino acid positions, respectively. B, basic; C, coiled-coil; G, GTP-binding; I, intermediate; A, acidic; HBD, homodimerization; UD, undefined domain. (D) Confocal images of GFP-fused wild-type and mutant TRF1 proteins in HeLa cells. Nucleoli are labeled by anti-B23 staining. Scale bar: 20 μm.