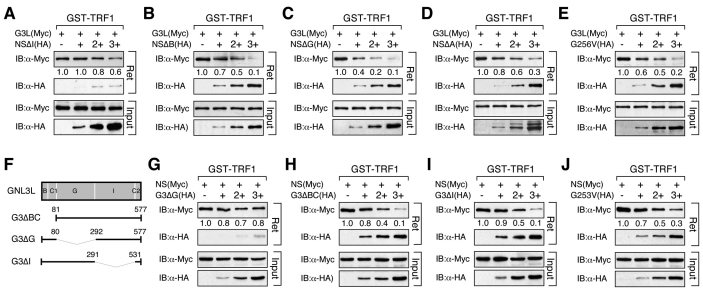
Fig. 3.
Identification of NS domain required for its ability to inhibit GNL3L binding to TRF1 and vice versa. Affinity binding between GST–TRF1 and GNL3L (Myc) was conducted in the presence of different NS deletion mutants. The NS ability to block GNL3L binding to TRF1 requires its TRF1-interacting I-domain (A), but is unaffected by any other deletions (B–D) or the G256V mutation (E). (F) GNL3L mutants used to map the domain(s) required for its competition with NS for TRF1 binding. The ability of GNL3L to block NS binding to TRF1 requires only its TRF1-interacting G-domain (G) and does not depend on its BC-domain (H), I-domain (I) or GTP binding (J).