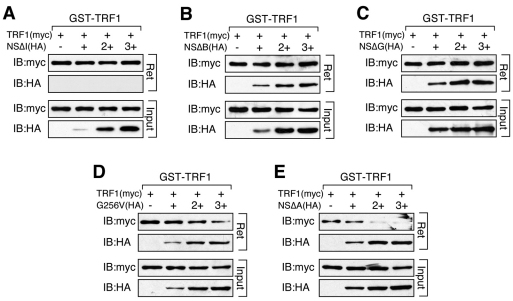
Fig. 4.
The anti-TRF1 dimerization effect of NS requires its basic and GTP-binding domains, in addition to its TRF1-binding I-domain. Agarose-bound GST–TRF1 was incubated with lysates containing a fixed amount of TRF1 (Myc) and increasing amounts of NS deletion mutants (HA). The NS ability to block TRF1 dimerization is completely abolished by deletions of its I-domain (A), B-domain (B) or G-domain (C), partially blocked by the GTP-binding mutant (G256V, D), but is completely unaffected by the A-domain deletion of NS (E). Agarose-bound (R) and supernatant fractions (S) are indicated.