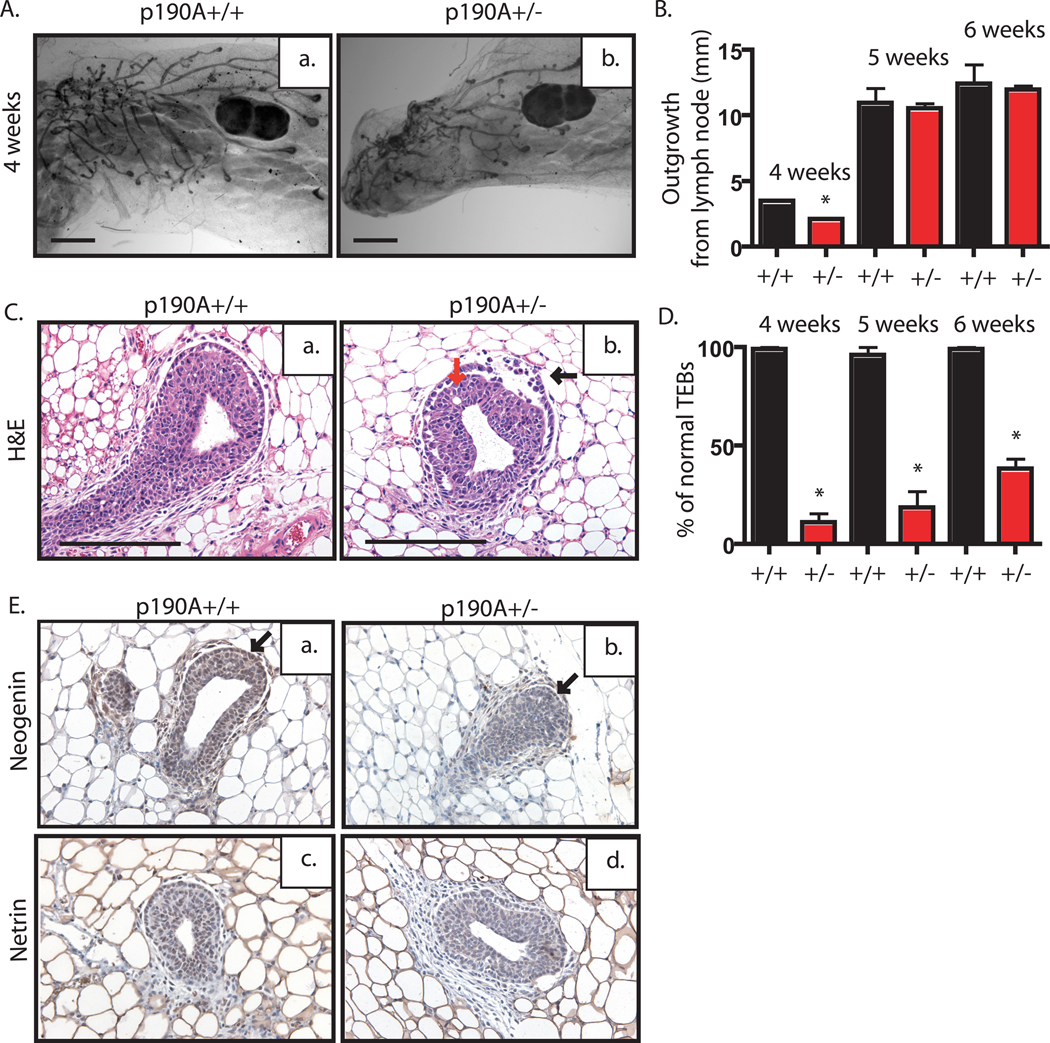
Figure 2. p190A heterozygosity, unlike p190B, does not delay virgin outgrowth but does disrupt TEB architecture.
A. Whole mounted mammary glands from sister pairs of 4-week-old wildtype (a) and p190A heterozygous (b) mice used to analyze the extent of ductal outgrowth. B. Using lymph node as a reference (indicated by LN), length of ductal outgrowth was recorded in millimeters (mm) for wildtype (black bar) and p190A heterozygous (red bar) (n=5 per genotype per time point). Plot indicates significantly reduced outgrowth at 4 week time point, only (p<.0001). C. Representative images of TEB from H&E-stained mammary gland tissue sections from wildtype (a) or p190A heterozygous (b) mice. Note the presence of dissociated cap cell layer (black arrow) and body cell layer (red arrow) in the p190A heterozygous TEB. D. The percentage of normal TEBs in wildtype (black bar) and p190A heterozygous (red bar) mice is depicted by a graph. Note the significant decrease in the number of normal TEBs at all time point (p<.0001). E. Representative images of immunohistochemical staining for neogenin and netrin. Neogenin expression appears to be decreased in the cap cell layer (arrows) of p190A heterozygous TEBs (b) compared to wildtype TEBs (a). Netrin staining intensity is similar in wildtype (c) and p190A heterozygous (d) TEBs.