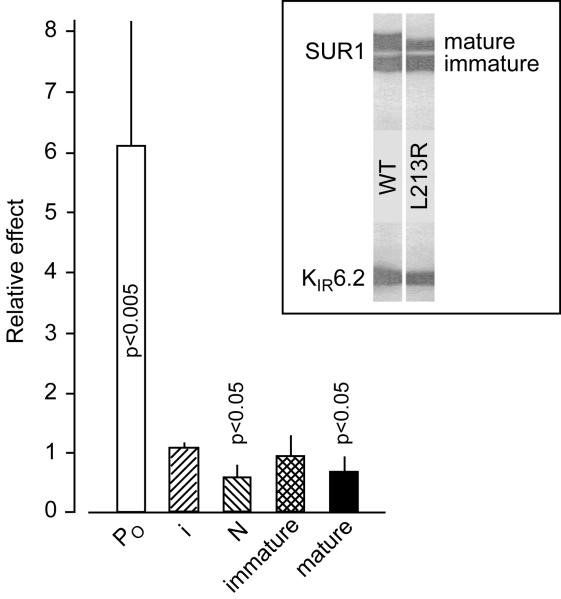
Fig. 2.
L213R markedly elevates on-cell PO, does not affect the unitary conductance, and slightly decreases functional expression of (SUR1/KIR6.2)4 complexes without altering their labeling with 1 nM 125I-azidoglibenclamide. Inset shows similar affinity photolabeling of immature, core-glycosylated SUR1L213R vs SUR1 with the second generation SU, and modestly reduced amount of mature, complex-glycosylated mutants trafficking with colabeled KIR6.2. As was reported earlier, nanomolar glibenclamide or micromolar tolbutamide abolish labelling by displacing the photoreactive analogue of glibenclamide which does not label KIR6.2 expressed without SUR1 [17,18,26], the L0 loop apparently coordinates the photoreactive group-containing non-sulfonylurea moiety of the drug [22,27], and deleting the N-terminus of KIR6.2 eliminates its colabeling without disrupting KATP assembly [18] required for trafficking of either SUR1 or glycosylation site-free KIR6.2 to the plasma membrane [26,28]. Therefore, normal affinity labeling of SUR1L213R and KIR6.2, while slightly reduced amount of mature SUR1L213R, suggest that the mutation does not alter the SU binding site or the direct proximity of L0 to the KIR N-terminal helix, while slightly reducing KATP trafficking to the plasma membrane; n ≥ 5 for each bar.