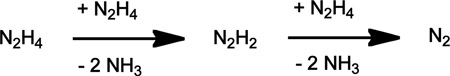Abstract
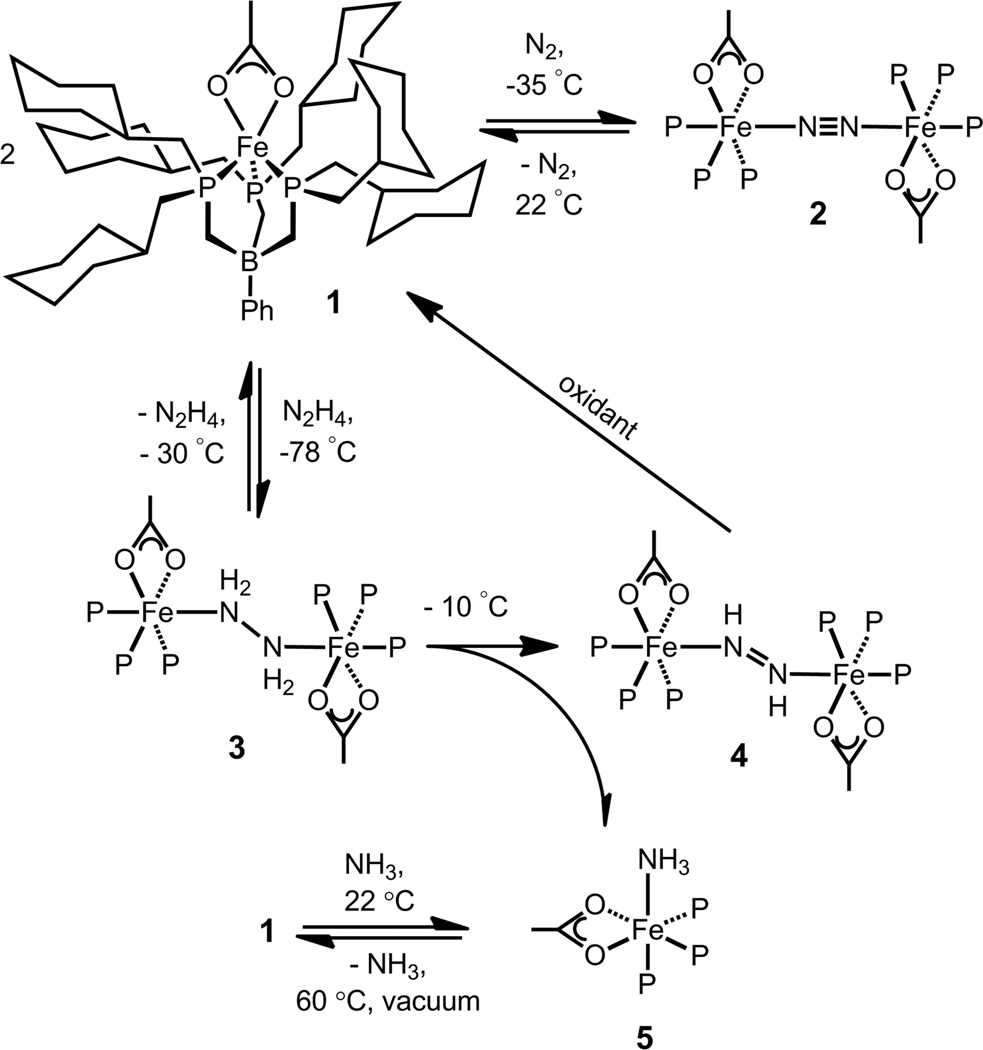
A family of iron(II) complexes that coordinate dinitrogen, diazene, hydrazine, and ammonia are presented. This series of complexes is unusual in that the complexes within it feature a common auxiliary ligand set and differ only by virtue of the nitrogenous NxHy ligand that occupies the sixth binding site. The ability of an iron center to bind N2, N2H2, N2H4, and NH3 is important to establish in the context of evaluating catalytic N2 reduction schemes that invoke these nitrogenous species. Such a scenario has been proposed as an iron-mediated, alternating reduction scheme within the cofactor of nitrogenase enzymes.
Owing to its biological and industrial relevance, establishing mechanisms for the reduction of N2 to NH3 is a longstanding goal of chemists.1 Several mechanisms have been proposed for a metal-mediated reduction, with the distal (i.e., Mn-N≡N → Mn+3≡N + NH3 → Mn + NH3) and alternating (i.e., Mn-N≡N → Mn-HN=NH → Mn-H2N-NH2 → Mn + 2 NH3) mechanisms representing two limiting schemes.1c,d,2 Schrock3 and Nishibayashi4 have respectively prepared mono- and di-molybdenum complexes that serve as catalysts for this transformation, the former of which is thought to proceed via a distal reduction scheme. To date, there are no examples of well-defined synthetic catalysts thought to proceed via the alternating reduction scheme, though it has been suggested that the biological reduction of N2 to NH3 at the FeMo-cofactor of nitrogenase may proceed via such a mechanism.5 Circumstantial support of such a hypothesis derives from the observation that both diazene and hydrazine are substrates for nitrogenase.1d,5 Also, spectroscopic studies of the FeMo-cofactor under turnover conditions appear to be consistent with an iron-bound NHy species, which is also observed in the reduction of diazene and hydrazine.5 Though the site of N2 coordination and subsequent reduction remains a matter of uncertainty, recent studies have suggested that N2 is reduced at one or more iron centers.1d,6 Hence, there is much interest in preparing synthetic iron complexes that coordinate NxHy ligands,7 as they serve as structural and spectroscopic models to the postulated trapped intermediates.
Mono- and di-iron complexes that coordinate N2Hy ligands (y = 2,3) remain relatively rare, and exhibit both acid/base and redox reactivity.7a–c,7e,f Despite this rich reactivity, there are no reported iron systems that can coordinate and/or interconvert a range of NxHy ligands that encompass the full range of sp, sp2, and sp3 hybridization at the nitrogen atoms, and the accompanying N-atom oxidations states 0, -1, -2, and -3.7g,h,8 Such systems are rare for any metal, with only two ruthenium9 and one manganese10 systems published in the literature. Herein we describe a series of iron complexes that coordinate N2, N2H2, N2H4, and NH3. These complexes feature identical auxiliary ligands, are all iron(II), and only differ in the extent that the nitrogenous ligand is reduced.
Access to the NxHy chemistry of present interest is realized using a 5-coordinate iron(II) complex, [PhBPCH2Cy3]Fe(OAc) (1) ([PhBPCH2Cy3] = PhB(CH2P(CH2Cy)2)3−). Complex 1 is quantitatively formed in the reaction between [PhBPCH2Cy3]FeMe and one equiv of AcOH, and is isolated as an analytically pure grey powder (Scheme 1). Five-coordinate 1 is paramagnetic with a room temperature solution magnetic moment of 4.5 μβ.
Scheme 1.
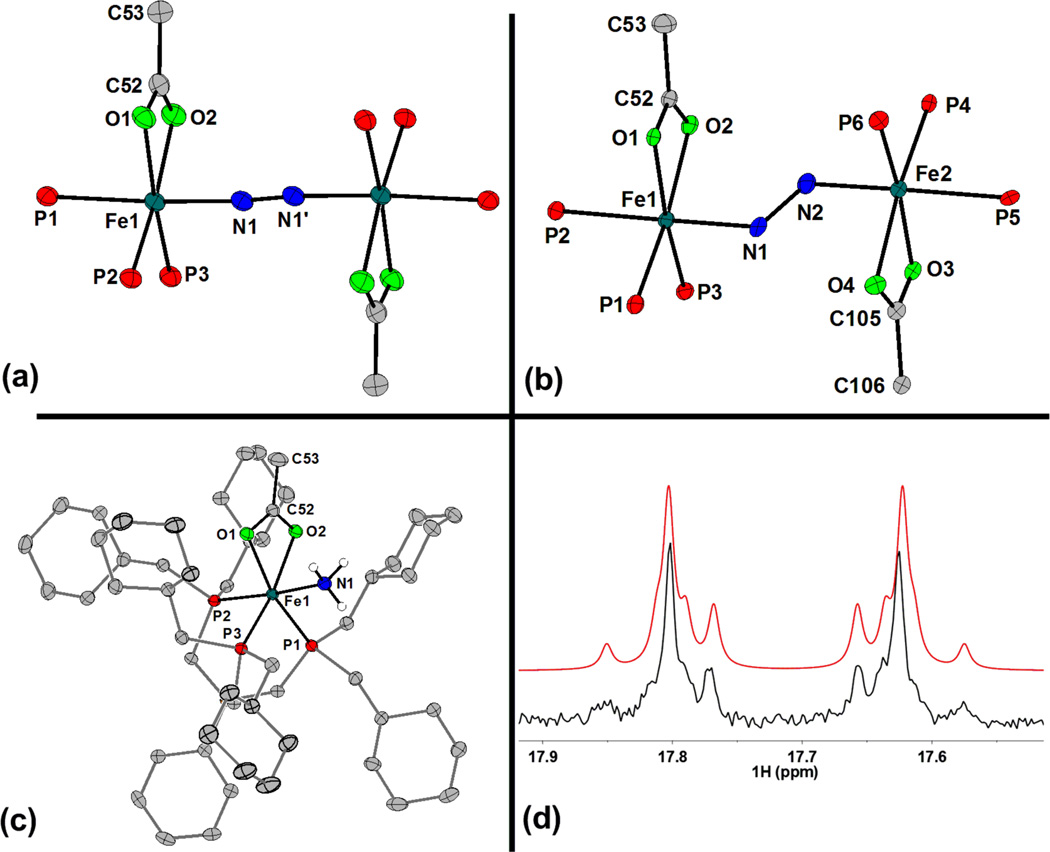
Complex 1 serves as a scaffold for which L-type ligands can bind, generating low-spin 6-coordinate mono- and diiron species. For example, cooling solutions of 1 under an N2 atmosphere results in the coordination of 0.5 equiv of N2 to generate the pink and diamagnetic μ-N2 species 2, {[PhBPCH2Cy3]Fe(OAc)}2(μ-N2). Crystals of 2 suitable for diffraction can be grown from saturated Et2O solutions of 2 stored at −35 °C in the glove-box, and its solid-state structure has been obtained (Figure 1a). The respective Fe-N and N-N distances of 1.874(3) and 1.120(5) Å indicate a small degree of N2 activation.11 Consistent with the small degree of N2 activation, a ν(NN) stretch is observed at 2083 cm−1 that shifts to 2010 cm−1 upon 15N-isotopic labeling (calc’d: 2012 cm−1).
Figure 1.
Displacement ellipsoid (50%) representation of (a) the core atoms of μ-N2 2, (b) the core atoms of μ-N2H2 4 (major component only), and (c) 5. Protons that were not located in the difference map have been removed for clarity. Select bond distances (Å) and angles (°) for 2: Fe1-P1 2.285(1); Fe1-P2 2.2319(9); Fe1-P3 2.2361(9); Fe1-O1 2.076(2); Fe1-O2 2.083(2); Fe1-N1 1.874(3); N1-N1’ 1.120(5); Fe1-N1-N1’ 174.9(3). Select bond distances (Å) and angles (°) for 4: Fe1-P1 2.219(3); Fe1-P2 2.311(4); Fe1-P3 2.240(4); Fe1-O1 2.082(8); Fe1-O2 2.110(7); Fe1-N1 1.902(8); Fe2-N2 1.898(8); N1-N2 1.31(1); Fe1-N1-N2 128.1(7); Fe2-N2-N1 129.0(7). (d) Diazene resonance in the 1H{31P} NMR spectrum (C6D6, 25 °C) of 15N-4 indicating the AA’XX’ splitting pattern (experimental, black; fit, red). The data was fit with MestReNova using the following parameters: δ = 17.72, 1JNH = −71.0 Hz, 2JNH = −1.1 Hz, 3JHH = 21.0 Hz, 1JNN = 12.0 Hz, linewidth = 3.5 Hz.
In solution, as in the solid-state, 2 exists as a diiron species; the 15N NMR spectrum of 15N-2 (THF-d8, −75 °C) shows a single resonance at 328.6 ppm, which is split into a doublet by the trans phosphine phosphorous atom (2JPN ≈ 15 Hz). Despite formation of a diiron species, the coordinated N2 ligand in 2 is labile, and warming solutions of 2 to room temperature regenerates 1.
Treatment of 2 with 0.5 equiv N2H4 at −78 °C generates the purple diiron species {[PhBPCH2Cy3]Fe(OAc)}2(μ-η1:η1-N2H4) (3) (Scheme 1). The presence of a single 15N NMR chemical shift for 15N-3 (δ = 103 ppm), coupled with a single NH2 resonance in the 1H NMR spectrum (δ = 2.51 ppm) indicates a bridging hydrazine ligand. As for 2, the hydrazine ligand is labile; at −30 °C resonances ascribed to both 1 and 3 are observed by 1H NMR spectroscopy.
The hydrazine species 3 is not thermally stable, and at −10 °C undergoes a disproportionation reaction to precipitate the dark blue diazene species {[PhBPCH2Cy3]Fe(OAc)}2(trans-μ-η1:η1-N2H2) (4) from solution, leaving the ammonia complex [PhBPCH2Cy3]Fe(OAc)(NH3) (5) (Scheme 1) in the supernatant.
The presence of a bridging trans diazene ligand in 4 is readily discerned by NMR spectroscopy. A resonance centered at 434 ppm is observed in the 15N NMR spectrum of 15N-4, indicative of a moderately activated diazene ligand.7e,f,12 In the corresponding 1H{31P} NMR spectrum, an AA’XX’ multiplet centered at 17.72 ppm is observed, consistent with the presence of a bridging diazene ligand (Figure 1d, see caption for fitting parameters). The relatively large 3JHH of 21.0 Hz suggests a trans ligation, and the magnitude of the 3JHH coupling in π-conjugated systems can further be used to infer bond distances;13 a linear relationship exists between 3JHH and the N-N bond distance for trans ligated diazene complexes (see SI). The observed 3JHH of 21.0 Hz suggests an N-N bond distance of ca. 1.31 Å in 4, between that expected for an N-N single and a double bond.
The solid-state structure of 4 was obtained, and the core atoms of the structure are shown in Figure 1b. The quality of the dataset is compromised by a total molecule disorder, in which 18 % of the molecules are translocated along the b-axis of the P2(1)/c unit cell. Though the diazene protons could not be located in the difference map, the structure confirms the trans diazene ligation. The average Fe-N-N angle of 128.5° is consistent with sp2-hybridized nitrogen atoms, and the metrical parameters about the Fe-NH-NH-Fe core are similar (within error) to those of other 6-coordinate diiron(II) bridging diazene complexes.7a,7eFurther, the N-N distance of 1.31(1) Å is in good agreement with that predicted from the 3JHH coupling constant.
Comparison of the structures for μ-N2 2 and μ-N2H2 4 indicates little reorganization of the auxiliary ligands of the iron centers and a net 2e−/2H+ difference in the nitrogenous ligand. In both structures, the acetate ligands reside on opposite faces of the dinuclear framework, and the Fe-O and Fe-P distances (that are cis to the N2Hy ligand) are nearly equivalent. Only the Fe-P distance to the phosphine that is trans to the N2Hy ligand significantly changes, with a ca. 0.02 Å elongation upon going from 2 to 4, consistent with a stronger trans influence exerted by diazene than dinitrogen.
Worth noting is that the trans diazene in 4 is prone to dissociation. Heating a toluene solution of 4 to 60 °C for 2 h results in complete transformation to acetate 1 and ammonia complex 5, the ammonia in the latter species presumably generated from the dis-proportionation of free diazene. In contrast, the cis diazene in the related complex, {[PhBPCH2Cy3]Fe}2(μ-η1:η1-N2H2)(μ-NH2)2 is stable in solution for days at 60 °C.7e As the cis isomer of free diazene is less stable than the trans isomer,14 the robustness observed in the latter species is likely due to the presence of additional bridging ligands that strengthen the fidelity of the bimetallic unit.
The disproportionation reaction of μ-N2H4 3 also generates the ammonia species 5, whose solid-state structure is shown in Figure 1c. This species can alternatively be prepared by the addition of excess NH3 to a THF solution of 1 (Scheme 1). Though 5 is stable in solution at room temperature, heating solutions of 5 under vacuum results in NH3 loss and formation of 1.
Hydrazine disproportionation reactions to generate diazene and ammonia have been observed at diruthenium9b,15 and diiron7d,e centers, and can be regarded as the first step in the disproportionation of hydrazine to dinitrogen and ammonia:
To determine whether μ-N2H2 4 further reacts with hydrazine to generate N2 and NH3, 0.95 equiv of 15N2H4 was added to a THF solution of 4, and both the reaction volatiles and residual solids were analyzed by NMR spectroscopy. 1H NMR analysis of the volatiles established the formation of 15NH3 (ca. 50 % yield) and no 14NH3. 1H and 31P NMR spectroscopy of the residual solids indicates that acetate 1 and ammonia 5 were the major iron containing products, with 14N-4 present as a minor species. These results are consistent with a disproportionation mechanism in which the bound diazene is oxidized to N2 by free N2H4, which itself is reduced to NH3.
Though 1 can serve as a hydrazine disproportionation catalyst (in the presence of 10 equiv of N2H4, a 16 % yield of NH3 is obtained), the reaction is hampered by ligand degradation; in addition to 1 and 5, (CH2Cy)2PMe and other, unidentifiable products are present in the resulting 1H and 31P NMR spectra.
The ability to isolate iron complexes that only differ in the extent of reduction of the nitrogenous ligand suggests the possibility that these complexes may be interconverted via redox reactions. Though the cyclic voltammogram of μ-N2 2 (obtained at −35 °C) shows a quasi-reversible reduction at ca. −2.2 V, the chemical reduction of 2 with [Na][C10H8] results in a mixture of unidentifiable products. Attempts to chemically reduce μ-N2 2 to μ-N2H4 4 at −78 °C with well-defined H-atom transfer agents (i.e. catechol, hydroquinone, Bu3SnH, cyclohexadiene and PhSH) or with combinations of reductants (i.e. Cp*2Fe, Cp*2Co, Cp2Co, [Na][C10H8]) and acids (i.e. HOAc, HOTf, [lutH][BPh4]), did not yield the desired transformation, and in most instances, 1 was the only identifiable iron containing species present (ligand degradation also occurred). In contrast, treatment of 4 with oxidants (e.g. p-benzoquinone, Pb(OAc)4) results in formation of 1, which may proceed through μ-N2 2. These results are perhaps not unexpected when one considers the respective gas-phase BDE of NN-H● (ca. 0 kcal/mol) and HNN-H● (60.8 kcal/mol).16 As the N2 is weakly activated in 2, addition of a net H-atom to 2 would likely generate a high energy and Fe2(μ-NNH●) intermediate that would decay back to 2. To circumvent such an intermediate, the direct reduction of 2 to 4 may call for a concerted 2e—/2H+ transfer.
In summary, a series of mono- and diiron(II) complexes that coordinate nitrogen, diazene, hydrazine, and ammonia in an available sixth coordination site have been prepared and characterized. These complexes are structurally related to one another by the ancillary ligands and differ only by the coordinated NxHy ligand, and hence present an attractive synthetic system for studying aspects of an alternating N2 reduction scheme. The finding that μ-N2H4 3 and μ-N2H2 4 react with free hydrazine, reducing the latter to NH3 as the former is oxidized, suggests that a similar reactivity pattern merits consideration in the reduction of diazene by nitrogenase.
Supplementary Material
ACKNOWLEDGMENT
We acknowledge the NIH (GM-070757). Funding for the Caltech NMR facility has been provided in part by the NIH (RR027690). C.T.S. is grateful for an NSF graduate fellowship.
Footnotes
ASSOCIATED CONTENT
Supporting Information. Detailed experimental procedures, characterization data for 1–5 (.pdf) and crystallographic details (2, 4, 5) (.cif) are included. This material is available free of charge via the Internet at http://pubs.acs.org.
REFERENCES
- 1.(a) MacKay BA, Fryzuk MD. Chem. Rev. 2004;104:385. doi: 10.1021/cr020610c. [DOI] [PubMed] [Google Scholar]; (b) Howard JB, Rees DC. Proc. Natl. Acad. Sci. U.S.A. 2006;103:17088. doi: 10.1073/pnas.0603978103. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Peters JC, Mehn MP. Bio-organometallic Approaches to Nitrogen Fixation Chemistry. In: Tolman WB, editor. Activation of Small Molecules. Wiley-VCH; 2006. p. 81. [Google Scholar]; (d) Hoffman BM, Dean DR, Seefeldt LC. Acc. Chem. Res. 2009;42:609. doi: 10.1021/ar8002128. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Crossland JL, Tyler DR. Coord. Chem. Rev. 2010;254:1883. [Google Scholar]
- 2.(a) Chatt J, Pearman AJ, Richards RL. J. Chem. Soc.;Dalton Trans. 1977:1852. [Google Scholar]; (b) Dilworth MJ, Thorneley RN. Biochem. J. 1981;193:971. doi: 10.1042/bj1930971. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) MacBeth CE, Harkins SB, Peters JC. Can. J. Chem. 2005;83:332. [Google Scholar]; (d) Hendrich MP, Gunderson W, Behan RK, Green MT, Mehn MP, Betley TA, Lu CC, Peters JC. Proc. Natl. Acad. Sci. U.S.A. 2006;103:17107. doi: 10.1073/pnas.0604402103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.(a) Yandulov DV, Schrock RR. Science. 2003;301:76. doi: 10.1126/science.1085326. [DOI] [PubMed] [Google Scholar]; (b) Schrock RR. Acc. Chem. Res. 2005;38:955. doi: 10.1021/ar0501121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arashiba K, Miyake Y, Nishibayashi Y. Nat Chem. 2011;3:120. doi: 10.1038/nchem.906. [DOI] [PubMed] [Google Scholar]
- 5.Lukoyanov D, Dikanov SA, Yang Z-Y, Barney BM, Samoilova RI, Narasimhulu KV, Dean DR, Seefeldt LC, Hoffman BM. J. Am. Chem. Soc. 2011 doi: 10.1021/ja2036018. ASAPS, 10.1021/ja2036018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.(a) Barney BM, Lukoyanov D, Igarashi RY, Laryukhin M, Yang TC, Dean DR, Hoffman BM, Seefeldt LC. Biochemistry. 2009;48:9094. doi: 10.1021/bi901092z. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Barney BM, Igarashi RY, Dos Santos PC, Dean DR, Seefeldt LC. J. Biol. Chem. 2004;279:53621. doi: 10.1074/jbc.M410247200. [DOI] [PubMed] [Google Scholar]
- 7.(a) Sellmann D, Sutter J. Acc. Chem. Res. 1997;30:460. [Google Scholar]; (b) Crossland JL, Zakharov LN, Tyler DR. Inorg. Chem. 2007;46:10476. doi: 10.1021/ic702007r. [DOI] [PubMed] [Google Scholar]; (c) Field LD, Li HL, Dalgarno SJ, Turner P. Chem. Commun. 2008:1680. doi: 10.1039/b802039f. [DOI] [PubMed] [Google Scholar]; (d) Chen Y, Zhou Y, Chen P, Tao Y, Li Y, Qu J. J. Am. Chem. Soc. 2008;130:15250. doi: 10.1021/ja805025w. [DOI] [PubMed] [Google Scholar]; (e) Saouma CT, Müller P, Peters JC. J. Am. Chem. Soc. 2009;131:10358. doi: 10.1021/ja903967z. [DOI] [PubMed] [Google Scholar]; (f) Saouma CT, Kinney RA, Hoffman BM, Peters JC. Angew. Chem. Int. Ed. 2011;50:3446. doi: 10.1002/anie.201006299. [DOI] [PMC free article] [PubMed] [Google Scholar]; (g) Field LD, Li HL, Magill AM. Inorg. Chem. 2009;48:5. doi: 10.1021/ic801856q. [DOI] [PubMed] [Google Scholar]; (h) Sellmann D, Soglowek W, Knoch F, Ritter G, Dengler J. Inorg. Chem. 1992;31:3711. [Google Scholar]
- 8.In ref. 7g, the authors report an Fe(η2-N2H2) diazene species. However, the structural and NMR parameters of this species (ref. 7c) are very similar to those of an Fe2(μ-η2-N2H22−) hydrazido(2-) species we have reported (ref. 7e), suggesting that this complex may be better described as a hydrazido(2-) rather than a diazene species.
- 9.(a) Collman JP, Hutchison JE, Lopez MA, Guilard R, Reed RA. J. Am. Chem. Soc. 1991;113:2794. [Google Scholar]; (b) Sellmann D, Hille A, Rösler A, Heinemann FW, Moll M, Brehm G, Schneider S, Reiher M, Hess BA, Bauer W. Chem. Eur. J. 2004;10 doi: 10.1002/chem.200305499. [DOI] [PubMed] [Google Scholar]
- 10.(a) Sellmann D. Z. Natureforsch. 1970;25 b:890. [Google Scholar]; (b) Sellmann D. Angew. Chem., Int. Ed. Engl. 1971;10:919. [Google Scholar]; (c) Sellmann D. J. Organomet. Chem. 1972;44:C46. [Google Scholar]
- 11.Field LD, Guest RW, Turner P. Inorg. Chem. 2010;49:9086. doi: 10.1021/ic101646p. [DOI] [PubMed] [Google Scholar]
- 12.(a) Smith MR, Cheng TY, Hillhouse GL. J. Am. Chem. Soc. 1993;115:8638. [Google Scholar]; (b) Bernskoetter WH, Pool JA, Lobkovsky E, Chirik PJ. J. Am. Chem. Soc. 2005:127–7901. doi: 10.1021/ja050387b. [DOI] [PubMed] [Google Scholar]
- 13.Cooper MA, Manatt SL. J. Am. Chem. Soc. 1969;91:6325. [Google Scholar]
- 14.Back RA, Willis C, Ramsay DA. Can. J. Chem. 1974;52:1006. [Google Scholar]
- 15.Kuwata S, Mizobe Y, Hidai M. Inorg. Chem. 1994;33:3619. [Google Scholar]
- 16.Warren JJ, Tronic TA, Mayer JM. Chem. Rev. 2010;110:6961. doi: 10.1021/cr100085k. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.