Abstract
Acute and prolonged bone complications associated with radiation and chemotherapy in cancer survivors underscore the importance of establishing a laboratory-based complementary dual-isotope tool to evaluate short- as well as long-term bone remodeling in an in vivo model. To address this need, a liquid scintillation dual-label method was investigated using different scintillation cocktails for quantitative measurement of 3H-tetracycline (3H-TC) and 45Ca as markers of bone turnover in mice. Individual samples were prepared over a wide range of known 45Ca/3H activity ratios. Results showed that 45Ca/3H activity ratios determined experimentally by the dual-label method were comparable to the known activity ratios (percentage difference ~2%), but large variations were found in samples with 45Ca/3H activity ratios in range of 2–10 (percentage difference ~ 20–30%). Urine and fecal samples from mice administered with both 3H-TC and 45Ca were analyzed with the dual-label method. Positive correlations between 3H and 45Ca in urine (R = 0.93) and feces (R = 0.83) indicate that 3H-TC and 45Ca can be interchangeably used to monitor longitudinal in vivo skeletal remodeling.
Keywords: 3H, 45Ca, liquid scintillator, scintillation cocktail, dual-label, bone remodeling
1. Introduction
1.1 Radioisotopes for Bone Remodeling Studies
Liquid scintillation has been used for the non-invasive study of bone remodeling with multiple radioisotopes (3H, 47Ca, 45Ca) (Bates et al., 1996; Fricke, 1975; Hanes et al., 1999; Mahin and Lofberg, 1966; Shahnazari et al.; Zhao et al., 2010). The method of dual radioisotope labeling in animals is widely used to verify (a) the same physiological process with different methods, or (b) two different physiological processes with respective radioisotopes in a single experiment. Literature is available for determination of dual radioisotopes viz. 32P and 45Ca (Bem and Reimschüssel, 1979), 3H and 14C (Los Arcos and Barquero, 1996; Reddy et al., 2009), 3H and 125I (Thibodeau et al., 1981) and 90Sr and 90Y (Lee et al., 2002). 3H-Tetracycline (3H-TC) (DeMoss and Wright, 1997) is individually used to quantify the resorptive phase of bone calcium metabolism while 45Ca is a bone seeking tracer generally used to investigate endocrine metabolism (Shahnazari et al.).
With a single intravenous injection, 3H-TC can best be used for short-term (days) kinetic studies due to its short biological half-life (~2.8 hours) (DeMoss and Wright, 1997), whereas 45Ca can be used for relatively longer-term (several months) kinetic studies due to its longer biological half-life (~187 days) (ICRP). Classically, 3H-TC is used to monitor bone resorption, because it is not readily incorporated into newly formed bone (Muhlbauer and Fleisch, 1990), whereas urinary excretion of calcium tracers is thought to reflect net bone turnover, because calcium tracer is reabsorbed through the kidney. Dual isotope studies have been conducted to compare calcium, the endogenous constituent of bone, with respect to 3H-TC, a known marker for bone resorption. For example, in animal models using 3H-TC and 45Ca (Zhao et al., 2010) and 3H-TC and 41Ca (Cheong et al., 2011), the authors show that both isotopes measured in urine can be used interchangeably to screen dietary and other interventions for beneficial effects on bone (Zhao et al., 2010). Due to the low kidney re-absorption upon turnover in animals, 3H-TC may also be a useful complementary assay technique to 45Ca for studying cancer treatment modalities (example, radiation) that may have a role in kidney damage. Finally, due to the much larger endogenous excretion of 45Ca in feces compared to urine in both mice and rats (Wang and Bhattacharyya, 1993; Zhao et al., 2010), feces can be used for a much longer period than urine to track bone turnover via 45Ca excreta measurements. To date, the kinetics of these two bone markers (3H-TC and 45Ca or 41Ca) have not been correlated in feces and urine.
1.2 Dual-Isotope Approach Using Liquid Scintillation Spectrometry
Liquid scintillation is a simple and expedited technique used for measuring radioisotopes. The important benefits are ease of sample preparation and high counting efficiency with low-levels of nuclide. The dual-isotope liquid scintillation technique can be highly beneficial, but the measurement is critically dependent on an accurate assessment of the actual radioactivity of both radioisotopes (Dodson, 2002). Because the β-particle energy is a continuum extending from zero to some maximum energy, the energy spectrum of a strong β-emitter may overlap the spectrum of a weak βemitter. Depending on the activity of both the radioisotopes and the beta energy difference, this overlap may lead to an error in the measurements. Additional difficulties include variable efficiency of one radioisotope compared to the other radioisotope at a particular energy, which may occur due to quench. Quench reduces the number of photons observed for a given amount of energy. With an increase in quench, the spectra generally shift towards the lower energy. Proper quench determination is crucial for accurate measurement of dual-labeled samples, as it helps in automatic detection of the shift.
Equally important is the use of an appropriate scintillation cocktail. The scintillation cocktails can be categorized by their phasing properties and ability to dissolve aqueous and non-aqueous samples. In the present study, we compared two categories of scintillation cocktails: cocktails that develop a gel phase with the sample (Universol™, Insta-Gel Plus and HI-Fluor) and cocktails that do not develop a gel phase with the sample (Ecolite™, Ecolume™).
Radioisotopes commonly used in biomedical research have low energy and a short range of air or fluid penetration. The liquid scintillation detection efficiency of radioisotopes in biological samples depends on direct contact with the scintillation cocktail and the sample (Medeiros et al., 2003). The liquid scintillation efficiency is in turn highly constrained by interferences such as inhomogeneity, chemiluminescence, phosphorescence, micro precipitation, adsorption, and chemical (impurities) or color quench, all of which are in a way related to the scintillation cocktail composition (Medeiros et al., 2003) and the sample preparation technique (L'Annunziata et al., 2003).
The three main objectives of the present liquid-scintillation-based study for dual radioisotope estimation in different biological samples were to investigate the (a) impact of different scintillation cocktails on dual-label radioisotope counting in different biological samples, (b) accuracy of the dual-label method in determining the actual amounts of both radioisotopes at known concentrations and (c) association of dual radioisotope excretion in urine and feces after in vivo administration of both bone markers to mice. Our aim in optimizing conditions to simultaneously quantitate both isotopes is to apply the dual-isotope approach to evaluate bone remodeling changes that occur in cancer patients after radiation and chemotherapy.
2. Materials and methods
2.1 Reagents and scintillation cocktails
The dual-label samples were prepared from 3H-tetracycline [7-3H(N)] (American Radiolabeled Chemicals Inc., St. Louis, MO) and 45CaCl2 (Perkin Elmer, Waltham, Massachusetts) solutions. These are beta emitting nuclides with average and maximum energy being respectively 5.7 keV and 18.6 keV for 3H and 77 keV and 257 keV for 45Ca. Five different types of scintillation cocktails were used, namely Ecolite™, Ecolume™ and UniverSol™ (MP Biomedicals, Irvine CA), and Insta-Gel Plus and HI-Fluor (Perkin Elmer, Boston, MA).
2.2 Apparatus
An alpha/beta liquid scintillation detector (Beckmann Coulter LS 6500) with an energy range of 0–2000 keV was used in the present study. It had a logarithmic amplification, a 32,768 channel multichannel analyzer (MCA) having an effective resolution of 0.0625 keV per channel (2000 keV/ 32,728 channels) and an automated background subtraction option. Different micro-pipettes (Eppendorf, CA) were used for precise measurement of 3H and 45Ca radioisotope solutions. Dilutions were conducted with deionized water (Fischer Scientific, NJ). All the liquid scintillation measurements were carried out in 20ml polyethylene scintillation vials (Perkin Elmer, MA).
2.3 Quench Calibration for 3H and 45Ca
The counting efficiency of 3H and 45Ca was determined by measuring a series of quenched standards for both the radioisotopes having a fixed dpm value. Quenched standards are generally prepared by adding variable amounts of a quenching agent, like nitromethane, to a fixed radioisotope of fixed activity. In the present study, an active stock solution of 45Ca containing 55,000 dpm/0.5 ml was prepared from the standard 45CaCl2 of 62.7 × 106 dpm/µl and deionized water. Then, 0.5ml of this active stock solution was added to 15ml of the Ecolite scintillation cocktail. After an initial precision measurement on 30 samples of 45Ca, a set of 10 samples were selected with less than ±1% deviation from the prepared dpm value of 55,000. A set of samples with a wide quenching range was then obtained by adding increasing volumes of nitromethane (0, 15, 30, 45, 60, 75, 90, 105, 120 and 135 µl) in those ten samples. For 3H radioisotope, the standard Kit (Lot #HGG0608, Perkin Elmer) containing 10 samples of ~50,000 dpm values and varying levels of quench was used.
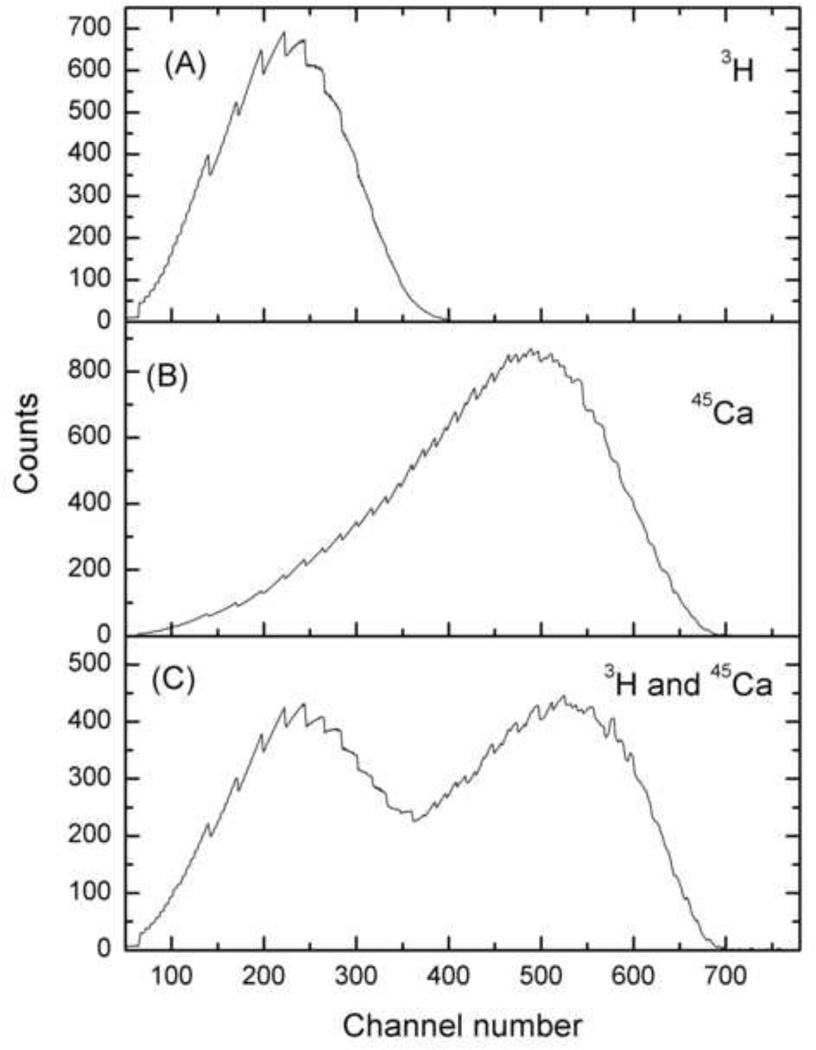
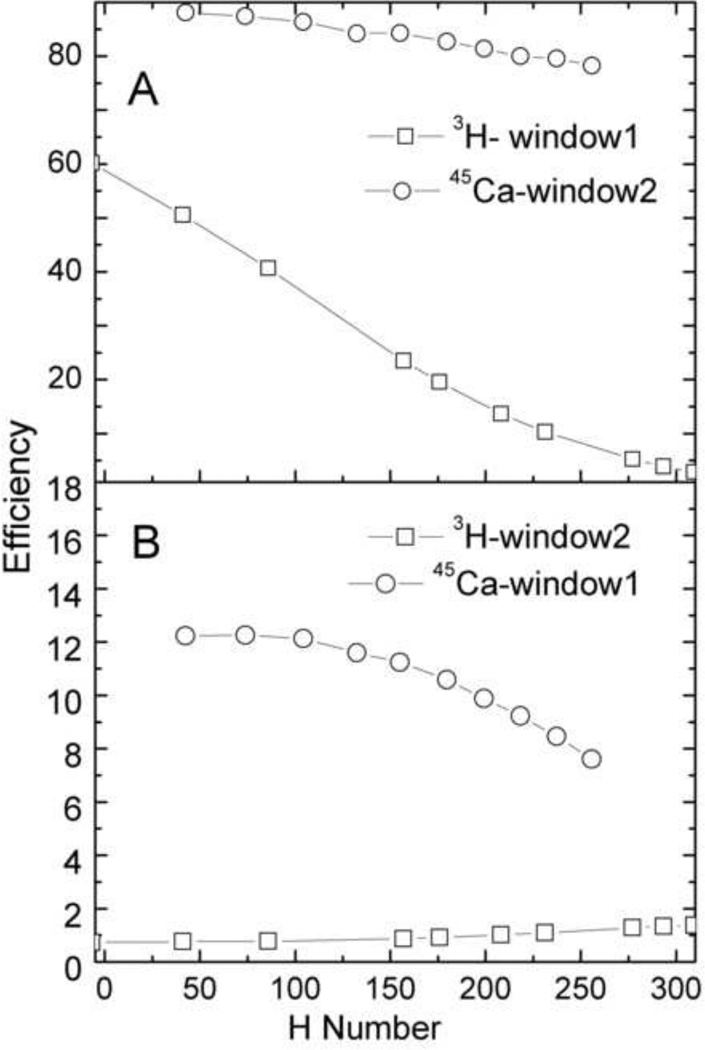
The quench-indicating parameter used by LSC 6500 is called the Horrocks number or the H#. This parameter is determined by calculation of the difference in channels between inflection points of the Compton edge of a quenched sample vs. an unquenched sample. The quench curve, or the plot of efficiency of the radioisotope as a function Horrocks number, was used for efficiency determination of both the radioisotopes. In dual-label mode, the quench curves were generated for each individual radioisotope in wide mode and then in two window settings viz. window1 and window2. Window1 mainly ranged from 0–15 keV and consisted of most of the beta spectra of 3H with a small tailing contribution of the 45Ca beta spectra. Window2 mainly ranged from 15–270 keV and consisted of mainly 45Ca beta spectra. LSC 6500 uses H# and a 32,768 channel multi-channel analyzer to provide an automated window adjustment as a function of quench using automatic quench compensation (AQC). This approach reduces the error from spill of the high energy isotope into the low energy isotope. An advantage of the use of H# is that any sample can have only one H#, which reflects the efficiency of counting the two radioisotopes in that sample. Fig. 1 shows the spectral distribution of a typical 3H and 45Ca radioisotope in single- and dual-label mode. The efficiency of 3H and 45Ca in both the single- and dual-label windows vs. H# is shown in Fig. 2.
Fig. 1.
The spectra of 3H and 45Ca in single and dual mode. Figure (A) is 3H spectra in single mode, figure (B) is 45Ca spectra in single mode, and figure (C) is 3H and 45Ca spectra in dual mode.
Fig. 2.
Efficiency (in %) of 3H and 45Ca as a function of H#. The upper figure (A) shows higher efficiency channel of the respective isotopes: the efficiency of 3H in window1 and 45Ca in window2. The lower figure (B) shows lower efficiency channel of the respective isotopes: the efficiency of 3H in window2 45Ca in window1.
2.4 Biological Sample Preparation
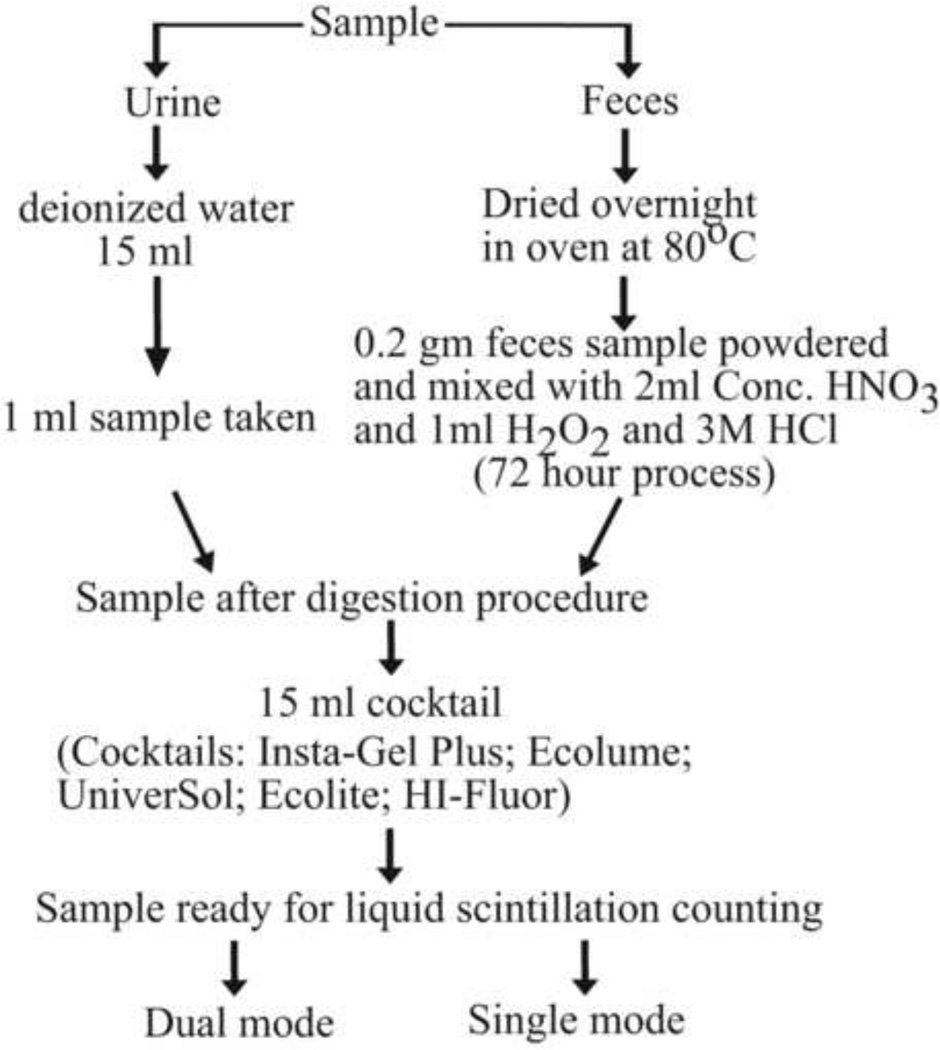
A total of 18 skeletally mature (15 weeks old) BALB/c mice were injected intravenously with 15µCi of 3H-tetracycline and 15µCi of 45CaCl2. This study was approved by the University of Minnesota Institutional Animal Care and Use Committee (IACUC). Following the tail vein IV injection, metabolic cages were used to collect 24 hour excreta (feces and urine) from each mouse individually at days 3, 6, 9, 13, and 16. Fecal samples for each mouse on a given collection day were transferred to pyrex beakers. The urine samples were retrieved by rinsing the cage with 15ml of deionized water. Feces digestion was a three day process. The oven-dried (80 °C) feces samples were weighed and powdered, and 0.2 g of that sample was solubilized in 2ml of conc. HNO3 on the first day, followed by second day addition of 1ml of hydrogen peroxide, and third day addition of 0.5ml 3M HCl and 1ml deionized water; the result was a clear, colorless solution. When assaying for 45Ca and 3H in all experiments, 0.5ml aliquots of feces and 1.0ml aliquots of urine samples were combined with 15ml of scintillation cocktail. The different sample preparation steps are schematically presented in Fig. 3. At least two sets of samples were prepared for each measurement of 3H and 45Ca, and dpm values were corrected for their respective half-life.
Fig. 3.
The flow diagram shows different sample preparation steps.
3. Experiment
3.1 Effect of different scintillation fluids
To check the activity of 3H and 45Ca in different biological samples, five scintillation cocktails were used, namely Ecolite™, Ecolume™, UniverSol™, Insta-Gel Plus and HI-Fluor. Each of the scintillation vials had 0.5ml of the digested biological sample solution and 15ml of scintillation cocktail. Measurements for different scintillation cocktails were carried out with their respective background sample. For example, while running the samples for Ecolite™, the background was made by adding 0.5ml of deionized water in 15ml of Ecolite™ followed by all the biological samples having only Ecolite™ as a scintillation cocktail. This process was repeated for the other scintillation cocktails. Each sample was counted for a period of 6 minutes.
3.2 Accuracy and sensitivity test for dual-label method
In this phase, the accuracy and sensitivity of the dual-label method was tested to determine the actual activity of 3H and 45Ca using the quench curve. Two groups of samples were prepared having the same or different activity combinations of 3H and 45Ca. For verification of the dual-label method, the samples were run in three different window settings-single, dual and wide window mode. The single-label mode was set for one radioisotope, 3H or 45Ca i.e. window1 or window2. The dual-label mode had two window settings for both 3H and 45Ca i.e. both window1 and window2 were used. In wide window mode, the full spectrum dpm was taken i.e. the whole scintillator window (0–2000 keV) was used. Steps involved in the verification process were as follows.
3.2.1
Multiple sets of single-isotope samples, containing either 3H or 45Ca, were prepared with activity (dpm) values from 2000–20,000 (e.g. 2000, 5000, 10000, 15000 and 20000 dpm). This range was chosen to make the test samples parallel the activities found in our biological samples. These single-isotope 3H and 45Ca samples were prepared in 10 ml of scintillation cocktail and were measured in both single and dual-label mode. The single mode was used to verify the individual strength, and the dual-label mode was used to determine the contribution (due to overlap) of one radioisotope into the respective window of the other radioisotope i.e. window2 for 3H and window1 for 45Ca. Multiple sets of samples were prepared for each isotope and activity value. These samples were run for ten repeat measurements. All the samples were kept in the same sequence and shaken before putting in the scintillator.
3.2.2
For each activity value, the vials with less than ±2% difference in dpm value were selected from multiple sets of samples. The selected vials were used to measure the dual radioisotope dpm values. The dual-isotope vials were prepared by mixing two single-isotope scintillation vials with different radioisotope solutions. The 45Ca/3H activity ratio in the resulting vials was varied from 0.1 to 10. These samples were run in dual-label and full spectrum modes.
3.2.3
The activity ratios of 45Ca: 3H obtained from the dual-label mode (Step 3.2.2) were compared with the activity ratios obtained by dividing the dpm values of the same single-isotope samples of 3H and 45Ca from the scintillation run conducted in single mode in Step 3.2.1.
3.3 Non-invasive dual radioisotope measurement in mice
As mentioned in section 2.4, 24-hour samples of urine and feces were collected from mice and pre-processed following the steps schematically presented in Fig. 3. The respective background was prepared by adding natural urine and feces samples (without the radioisotope) to the 15ml of Ecolite™. A scatter plot was drawn to measure the association (correlation coefficient) between 3H and 45Ca in the mouse urine and feces samples collected from days 3 to 16 post radiolabelling.
4. Results
In the present work, we measured the efficiency of 3H and 45Ca in single and dual-label mode. This was done by determining the quench curve in two separate window settings for low energy (3H) and high energy (45Ca) radioisotopes. As shown in Fig. 2, for equivalent quenching ranges, the H# and counting efficiency of 3H respectively ranged from 40–315 and 4.5–62%. The maximum efficiency of 3H was 62% in window 1. Likewise, for 45Ca measurements, the H# and counting efficiency respectively ranged from 47–247 and 87–98%. The maximum efficiency of 45Ca was 98% in window 2.
Table 1 shows the comparison of 3H and 45Ca dpm values in urine and feces samples in different scintillation cocktails. In Table 1, the results were compared as percentage difference from the average dpm obtained with Ecolite™. Overall, the 45Ca dpm values were found comparable in all the scintillation cocktails (percentage difference ≤4%) except HI-Fluor (percentage difference ≤16%). The total 3H dpm values of urine samples were also found to be similar (percentage difference ≤5%). However, for the feces samples, dpm values of 3H were higher in Universol™ (~ 14%) and HI-Fluor (~ 63 %) and lower in UniverSol™ (~ 14%); in the case of Insta-Gel Plus, the feces dpm values were comparable to Ecolite™ (percentage difference ~ 3%).
Table 1. Comparison between full spectrum dpm values.
Comparison between full spectrum dpm values with different scintillation cocktails. The average error in the measurements was ±5%.
| Sample | Ecolite | Ecolume | UniverSol (US) |
Insta-Gel Plus (IG) |
HI- Fluor (HIF) |
Percentage difference in Ecolite dpm values from |
|||
|---|---|---|---|---|---|---|---|---|---|
| Ecolume | US | IG | HIF | ||||||
| 3H (dpm) | |||||||||
| Urine | 4746 | 4554 | 4799 | 4631 | 4445 | 4.0 | −5.4 | 3.5 | 4.0 |
| Feces | 2811 | 2425 | 2760 | 2848 | 4650 | 13.7 | −13.8 | −3.2 | −63.3 |
| 45Ca (dpm) | |||||||||
| Urine | 805 | 781 | 810 | 805 | 767 | 3.0 | −3.7 | 0.6 | 4.7 |
| Feces | 6631 | 6595 | 6723 | 6595 | 5519 | 0.5 | −1.9 | 1.9 | 16.3 |
The single-isotope 3H and 45Ca samples run in dual-label mode showed negligible contribution in their low efficiency window (i.e. the window of the other radioisotope). The feeding of pure 45Ca in the 3H window i.e. window1 was less than 2%, while in the case of pure 3H radioisotope, no contribution was found in the 45Ca window i.e. window 2. Table 2 shows the percentage difference in the activity ratio of isotopes in each dual radioisotope sample from the actual activity ratio, which was calculated from the results of counting the samples individually in single mode before mixing the two radioisotopes. At the same activity value of both the radioisotopes, the percentage difference was found to be ≤ 4%. A slightly larger percentage difference was found at the high dpm value of both 3H and 45Ca (20,000 dpm). For most of the other samples, the percentage difference varied from 2%. However, large percentage differences (~13% to ~28%) were found in the case of samples having higher concentrations of 45Ca in the mixture sample (i.e. higher 45Ca/3H ratios). Compared to the case of the higher concentrations of 45Ca, the higher concentrations of 3H (i.e., lower 45Ca/3H ratio) gave lesser variations (≤ 7%).
Table 2. Comparison of 3H and 45Ca dpm values obtained in single.
Comparison of 3H and 45Ca dpm values obtained in single vs. dual-label mode. Pairs of samples containing prepared amounts of either 3H or 45Ca were counted in single-label mode, then mixed and recounted in dual-label mode. Maximum difference in samples with equal activities of the two isotopes was ±3%.
| Single-label mode | Dual-label mode | % difference in 45Ca/3H in dual-label mode and single-label mode |
||||
|---|---|---|---|---|---|---|
| 3H | 45Ca | 45Ca/3H | 3H | 45Ca | 45Ca/3H | Dual mode |
| 1961 | 1958 | 1.00 | 1895 | 1818 | 0.96 | 3.90 |
| 5034 | 5025 | 1.00 | 4792 | 4568 | 0.95 | 4.51 |
| 10093 | 9925 | 0.98 | 9482 | 8983 | 0.95 | 3.66 |
| 15222 | 15278 | 1.00 | 14261 | 13818 | 0.97 | 3.46 |
| 20136 | 20286 | 1.01 | 19352 | 18218 | 0.94 | 6.56 |
| 1915 | 19938 | 10.41 | 2403 | 17998 | 7.49 | 28.0 |
| 5030 | 20107 | 4.00 | 5258 | 18302 | 3.48 | 12.9 |
| 9945 | 20365 | 2.05 | 11305 | 18180 | 1.61 | 21.5 |
| 15114 | 19912 | 1.32 | 12951 | 18103 | 1.40 | −6.10 |
| 20177 | 2082 | 0.10 | 18607 | 1887 | 0.10 | 1.71 |
| 19410 | 4968 | 0.26 | 18505 | 4502 | 0.24 | 4.93 |
| 20567 | 9896 | 0.48 | 19442 | 8981 | 0.46 | 3.99 |
| 19417 | 15169 | 0.78 | 18802 | 13674 | 0.73 | 6.90 |
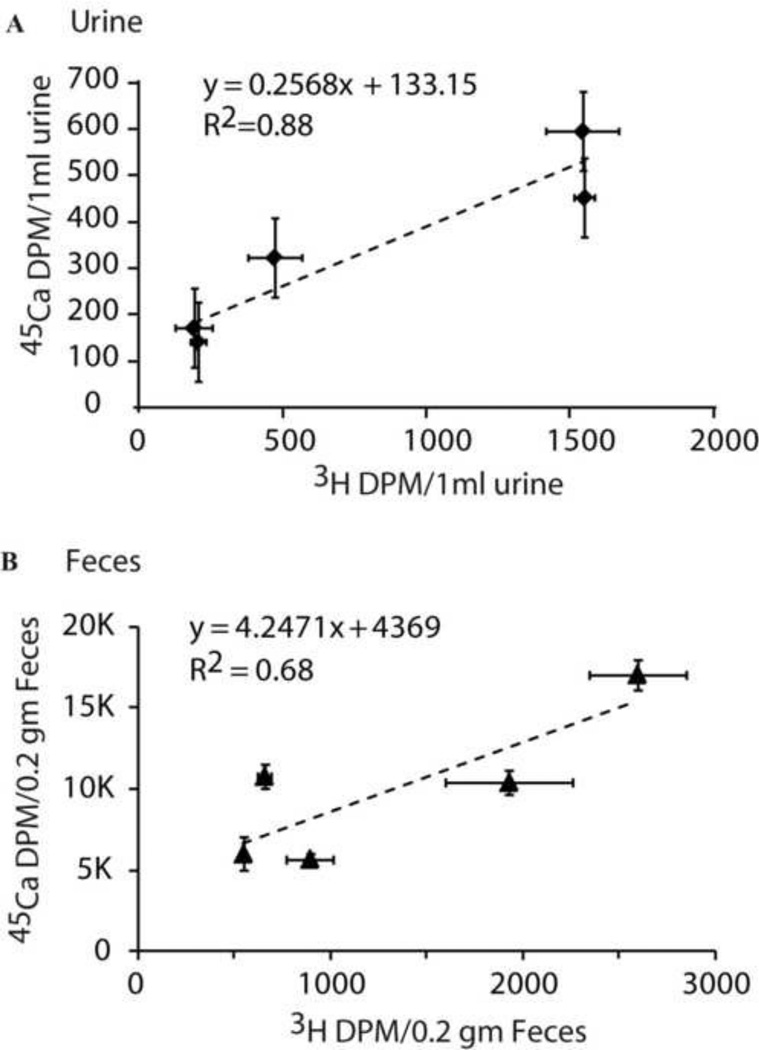
In the mouse study, rapid excretion of 45Ca and 3H was observed early after administration, followed by decreasing excretion in feces and urine. Fig. 4 shows the correlation between 3H and 45Ca dpm values for urine and feces samples collected during day 3 to 16 days. These values were obtained using Ecolite™ scintillation cocktail. The 45Ca:3H ratios ranged from 0.29–0.90, indicating that the dpm values in Fig 4 should be accurate within 7% (Table 2). Finally, results showed a positive linear correlation between 3H and 45Ca, with correlation coefficients (R) of 0.93 and 0.83 for urine and feces, respectively.
Fig. 4.
The scatter plot of 3H and 45Ca activity in mouse urine/1ml (A) and mouse feces/0.2gm (B) from day 3 to day 19 after administration of isotopes. Error bars show ± SEM for each data point.
5. Discussion
Proper control of certain parameters in liquid scintillation counting, such as the quench curve and the scintillation cocktail, might reduce the error involved in the accurate determination of activity. In the selection of an optimal liquid scintillation cocktail, the important aspects generally taken into consideration are overall cocktail performance and specific laboratory needs (Verrezen et al., 2008). Ecolite™ is generally used for a wide variety of biological samples, Ecolume™ is considered good for high ionic salts while Universol™ is good for insoluble and particulate samples. The other two scintillation cocktails used were Insta-Gel Plus and HI-Fluor. The former has high concentrated salt tolerance, fast chemiluminescence decay and high quench resistance. The latter is considered good for salt samples with high efficiency and low background. Although the dpm values in Insta-Gel Plus and HI-Fluor were higher, we preferred Ecolite™ in the present study because it had additional qualities such as 1) it did not make a gel phase with the sample, 2) it was bio-degradable and 3) it could be used easily for both aqueous and non-aqueous samples. The other scintillation cocktail Ecolume™ would also have been useful for a similar study.
The usual analytic methods for dual radioisotope studies take advantage of large energy differences between the radioisotopes. Although there is a sufficient difference between the β energies emitted by 3H (Eav = 5.7 keV, Emax = 18.6 keV) and 45Ca (Eav = 77 keV, Emax = 252 keV), the internal bremsstrahlung effect of the 45Ca spectra (Babu et al., 1976) emitted in the β-decay may lead to sufficient tailing of the peak. This may lead to fictitious peak area measurement in the 3H window and hence lead to inaccurate results, especially for higher activity concentrations of 45Ca compared to 3H. So for better quantification of radionuclides in the dual-label mode, it is crucial to verify the effect of 45Ca in the 3H regions, especially with increasing quench. In the present measurements, we found that the amount of overlap of the 45Ca β spectra in the 3H region was relatively low except at higher relative concentrations of 45Ca. The present study suggests that, for dual-labeled biological samples having higher activity differences between 3H and 45Ca, initial measurements should be made to evaluate the contribution of 45Ca in the 3H window. The latter consideration is particularly important to the mouse model in this study, because a significant increase in the 45Ca:3H ratio in excreta occurs with time after administration of the two bone markers, due to the fact that the biological half-life for 45Ca (~187 d) is so much longer than for 3H-TC (~2.8 hr). But, for the samples having higher activity of 3H compared to 45Ca, such preliminary measurements should not be needed. The close comparison between the actual and observed dpm values in Table 2 validates the effectiveness of the dual-label mode at other activity ratios.
Finally, applying the dual-isotope method to the in vivo experiment, we found a high correlation between 3H and 45Ca in mice excreta, both urine and feces. Therefore, 3H-tetracycline and 45Ca may be interchangeably used to monitor temporal bone remodeling responses, using either feces or urine. Cheong et al. observed a correlation between 45Ca in urine and in bone (Cheong et al., 2009). However, unlike 3H-TC, the majority (80–90%) of endogenous 45Ca excretion occurs via feces in mice (Wang and Bhattacharyya, 1993), making feces an important sample to monitor in that species. It has been recently shown that 41Ca can be used for short- and long-term (6 years) monitoring of bone remodeling in humans (Denk et al., 2007; Fitzgerald et al., 2005; Hui et al., 2007). As shown by Elmore et al. in dogs, 45Ca can serve as a surrogate marker of 41Ca (Elmore et al., 1990). 45Ca is a preferred marker in non-human animal models because it is easy to measure and less expensive.
The in vivo method established with this work provides the foundation for highly significant follow-on studies that use this radioisotope model to mimic clinical scenarios. For example, we are preparing to apply this model to gain insight into the acute (short-term) and prolonged (long-term) bone complications associated with radiation and chemotherapy in cancer survivors, which is a growing concern due to the aging population and the increased numbers of cancer survivors.
6. Conclusions
In the present study, we found that Ecolume™ and UniverSol™ are comparable to Ecolite™ for different biological sample digestion processes. Moreover, the dual-label method can be used for accurate estimation of 3H and 45Ca in different biological samples. For high concentrations of 45Ca compared to 3H, it is important to evaluate the contribution of 45Ca in the 3H window. 3H-TC or 45Ca can be used to monitor temporal bone remodeling responses via measurements in murine excreta.
Research Highlight.
Liquid scintillation cocktails support accurate dual-label analysis of 3H and 45Ca
3H-tetracycline and 45Ca were highly correlated in mice urine and feces.
3H-tetracylcine and 45Ca can be used to monitor in vivo temporal bone remodeling
Acknowledgement
This work is supported by the University of Minnesota's Masonic Cancer Center, Joseph E. Wargo Cancer Research and the National Institute of Child Health and Human Development (NICHD) (1K12-HD055887-01) through the BIRCWH (Building Interdisciplinary Careers in Women's Health) program. This work was also supported by PHS Cancer Center Support Grant P30 CA77398 and the NIH grant (R03 AR055333-03).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Babu R, Murty K, Murty V. Internal bremsstrahlung spectral shapes of 45Ca and 35S. Journal of Physics G: Nuclear Physics. 1976;2:331–339. [Google Scholar]
- Bates PI, Sharma HL, Murrer BA, McAuliffe CA. The tissue distribution in BALB/c mice of C-14-labeled JM216, an orally active platinum antitumour compound. Cancer Chemotherapy and Pharmacology. 1996;39:170–175. doi: 10.1007/s002800050555. [DOI] [PubMed] [Google Scholar]
- Bem H, Reimschüssel W. Simultaneous determination of phosphorus-32 and calcium-45 activity in biological samples. Journal of Radioanalytical and Nuclear Chemistry. 1979;52:361–367. [Google Scholar]
- Cheong J, Gunaratna N, McCabe G, Jackson G, Weaver C. Bone Seeking Labels as Markers for Bone Turnover: Effect of Dosing Schedule on Labeling Various Bone Sites in Rats. Calcified Tissue International. 2009;85:444–450. doi: 10.1007/s00223-009-9285-z. [DOI] [PubMed] [Google Scholar]
- Cheong JMK, Gunaratna N, McCabe G, Jackson G, Kempa-Steczko A, Weaver C. Bone-seeking labels as markers for bone turnover: validation of urinary excretion in rats. Osteoporosis international. 2011;22:153–157. doi: 10.1007/s00198-010-1281-7. [DOI] [PubMed] [Google Scholar]
- DeMoss DL, Wright GL. Analysis of whole skeleton 3H-tetracycline loss as a measure of bone resorption in maturing rats. Calcif Tissue Int. 1997;61:412–417. doi: 10.1007/s002239900357. [DOI] [PubMed] [Google Scholar]
- Denk E, Hillegonds D, Hurrell RF, Vogel J, Fattinger K, Hauselmann HJ, Kraenzlin M, Walczyk T. Evaluation of 41calcium as a new approach to assess changes in bone metabolism: effect of a bisphosphonate intervention in postmenopausal women with low bone mass. J Bone Miner Res. 2007;22:1518–1525. doi: 10.1359/jbmr.070617. [DOI] [PubMed] [Google Scholar]
- Dodson C. The scintillation counter. USA: Oxford University Press; 2002. [Google Scholar]
- Elmore D, Bhattacharyya MH, Sacco-Gibson N, Peterson DP. Calcium-41 as a long-term biological tracer for bone resorption. Nuclear Instruments and Methods in Physics Research Section B: Beam Interactions with Materials and Atoms. 1990;52:531–535. [Google Scholar]
- Fitzgerald RL, Hillegonds DJ, Burton DW, Griffin TL, Mullaney S, Vogel JS, Deftos LJ, Herold DA. 41Ca and Accelerator Mass Spectrometry to Monitor Calcium Metabolism in End Stage Renal Disease Patients. Clinical Chemistry. 2005;51:2095–2102. doi: 10.1373/clinchem.2005.049650. [DOI] [PubMed] [Google Scholar]
- Fricke U. Tritosol: A new scintillation cocktail based on Triton X-100. Analytical Biochemistry. 1975;63:555–558. doi: 10.1016/0003-2697(75)90379-6. [DOI] [PubMed] [Google Scholar]
- Hanes DA, Weaver CM, Wastney ME. Calcium and Oxalic Acid Kinetics Differ in Rats. Journal of Nutrition. 1999;129:165–169. doi: 10.1093/jn/129.1.165. [DOI] [PubMed] [Google Scholar]
- Hui SK, Prior J, Gelbart Z, Johnson RR, Lentle BC, Paul M. A pilot study of the feasibility of long-term human bone balance during perimenopause using a 41Ca tracer. Nuclear Inst. and Methods in Physics Research, B. 2007;259:796–800. [Google Scholar]
- ICRP, I. Limits for Intakes of Radionuclides by Workers. Vol. 30. ICRP Publication; 1980. pp. 3–4. [Google Scholar]
- L'Annunziata M, El Baradei M, Burkart W. Handbook of radioactivity analysis. Academic Pr.; 2003. [Google Scholar]
- Lee M, Chung K, Choi G, Lee C. Measurement of 90Sr in aqueous samples using liquid scintillation counting with full spectrum DPM method. Applied Radiation and Isotopes. 2002;57:257–263. doi: 10.1016/s0969-8043(02)00108-2. [DOI] [PubMed] [Google Scholar]
- Los Arcos J, Barquero R. Low-level assay of 3H and 14C MDA limits with conventional liquid scintillation counters using the LOLES' procedure. Applied Radiation and Isotopes. 1996;47:879–883. [Google Scholar]
- Mahin DT, Lofberg RT. A simplified method of sample preparation for determination of tritium, carbon-14, or sulfur-35 in blood or tissue by liquid scintillation counting. Analytical Biochemistry. 1966;16:500–509. [Google Scholar]
- Medeiros R, Godinho R, Mattos M. Comparison of the efficacy of biodegradable and non-biodegradable scintillation liquids on the counting of tritium-and [14C]-labeled compounds. Brazilian Journal of Medical and Biological Research. 2003;36:1733–1739. doi: 10.1590/s0100-879x2003001200016. [DOI] [PubMed] [Google Scholar]
- Muhlbauer RC, Fleisch H. A method for continual monitoring of bone resorption in rats: evidence for a diurnal rhythm. American Journal of Physiology- Regulatory, Integrative and Comparative Physiology. 1990;259:679–689. doi: 10.1152/ajpregu.1990.259.4.R679. [DOI] [PubMed] [Google Scholar]
- Reddy P, Bhade S, Narayan K, Narayanan A, Babu D, Sharma D. Comparative study of different methods for the activity quantification of 3H and 14C radionuclides in dual labeled samples using liquid scintillation analyzer. Applied Radiation and Isotopes. 2009;67:1945–1951. doi: 10.1016/j.apradiso.2009.07.006. [DOI] [PubMed] [Google Scholar]
- Shahnazari M, Burr D, Lee W, Martin B, Weaver C. Cross-calibration of 45calcium kinetics against dynamic histomorphometry in rats to determine bone turnover. Bone. 2010;46:1238–1243. doi: 10.1016/j.bone.2010.02.003. [DOI] [PubMed] [Google Scholar]
- Thibodeau S, Freeman L, Jiang N. Simultaneous measurement of estrogen and progesterone receptors in tumor cytosols with use of 125I-labeled estradiol and of 3H-R5020. Clinical Chemistry. 1981;27:687. [PubMed] [Google Scholar]
- Verrezen F, Loots H, Hurtgen C. A performance comparison of nine selected liquid scintillation cocktails. Applied Radiation and Isotopes. 2008;66:1038–1042. doi: 10.1016/j.apradiso.2008.02.050. [DOI] [PubMed] [Google Scholar]
- Wang C, Bhattacharyya M. Effect of cadmium on bone calcium and 45Ca in nonpregnant mice on a calcium-deficient diet: evidence of direct effect of cadmium on bone. Toxicology and applied pharmacology. 1993;120:228–239. doi: 10.1006/taap.1993.1107. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Cheong JMK, Lee WH, Wastney M, Martin BR, Weaver CM. Tetracycline and Calcium Kinetics Are Comparable for Estimating Bone Resorption in Rats. The Journal of nutrition. 2010;140:1704. doi: 10.3945/jn.110.121301. [DOI] [PMC free article] [PubMed] [Google Scholar]