Figure 3. Two classes of mechanisms that may control AMPAR positioning in the synapse.
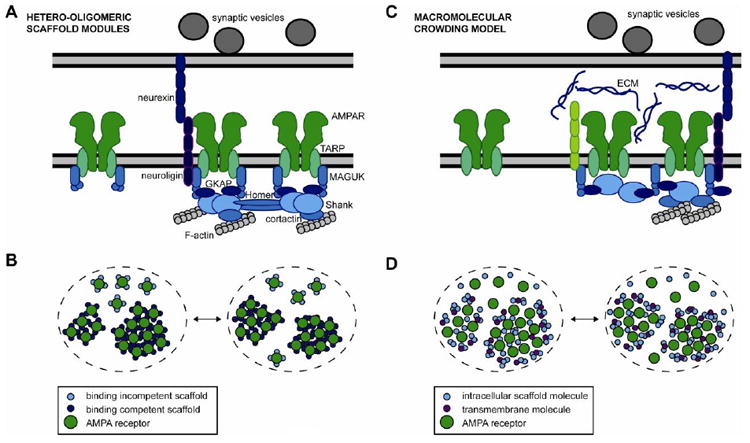
(A) Side view of a synapse, showing hetero-oligomeric scaffold modules controlling the position of AMPARs by direct protein-protein interactions. In the binding model, AMPAR/TARP complexes are stably anchored by interacting with MAGUK proteins such as PSD-95. The multimeric MAGUK proteins and other scaffold molecules can engage in multiple interactions with each other, and can assemble into hetero-oligomeric scaffold modules. PSD-95 forms complexes with GKAP and Shank molecules that interact with the actin cytoskeleton via cortactin and other intermediates. Intramolecular interactions between the SH3 and GK domains of MAGUKs potentially control oligomerization of numerous components of scaffold modules, as well as signaling molecules (not shown). Drawings are not to scale. (B) Top view of a synapse, showing hetero-oligomeric scaffold modules organizing the lateral distribution of AMPARs in the synapse. Modification of the binding competency of scaffolding molecules can change their oligomerization capability (light vs. dark blue small circles) and regulate interactions with AMPARs over time. (C) Side view of a synapse, showing different sources of macromolecular crowding. Scaffolding molecules close to the membrane can reduce AMPAR mobility, even if binding to receptors is rare. Postsynaptic transmembrane proteins such as neuroligin and cadherins may serve as obstacles to receptor motion, or alternatively may form structures more reminiscent of fences that subdivide the PSD. Transmembrane or extracellular proteins contribute to macromolecular crowding in the synaptic cleft, where the bulky extracellular domain of the AMPAR may increase obstruction of receptor motion. (D) Macromolecular crowding by various proteins (small circles) as in C can retain receptors within the synapse and within synapse subdomains over time. Note that the same distribution of receptors as in B could be produced by a differential distribution of obstructions. Both mechanisms shown in B and D, macromolecular crowding and binding to hetero-oligomeric scaffold modules, likely operate in tandem to determine receptor lateral distribution.
