Abstract
The disposition of ceftriaxone was studied after a single 2 g intravenous dose in seven patients 3 to 5 days after liver transplantation. Ceftriaxone concentrations in plasma, urine, and bile were measured by HPLC, and plasma protein binding was determined by equilibrium dialysis. Plasma protein binding was nonlinear, and the unbound fraction varied between 0.05 and 0.56. Both capacity and affinity were markedly different from reported values for normal subjects. The pharmacokinetic parameters obtained were: total body clearance (TBC), 11.2 ± 7.8 mL/hr/kg total and 44.8 ± 29.1 mL/hr/kg unbound; volume of distribution (Varea), 224 ± 76 ml/kg total and 767 ± 432 ml/kg unbound; steady-state volume of distribution (Vss), 212 ± 68 mL/kg total and 651 ± 368 mL/kg unbound; terminal disposition half-life (t1/2), 21.6 ± 14.3 hour total and 16.3 ±11.1 hour unbound. TBC for both total and free drug was considerably lower than literature values for normal subjects. Varea for total drug was greater than normal, whereas the corresponding value for free drug was smaller than normal. The plasma ceftriaxone concentrations at 12 and 24 hours were above the reported minimum inhibitory concentration (MIC). The fraction of the administered dose excreted in urine over 24 hours was 38 ± 29% and did not differ markedly from that reported for normal subjects. Less than 2% of the administered dose was excreted in 24-hour bile; however, biliary concentrations were always above MIC. Ceftriaxone can be administered once or twice daily at a dose of 2 g/day for prophylaxis in liver transplant recipients.
Ceftriaxone is a third-generation, parenteral cephalosporin with broad-spectrum activity against gram-positive and gram-negative pathogens.1 At the University of Pittsburgh, cefotaxime, another third-generation cephalosporin, is currently used for prophylaxis before and for 5 days after orthotopic liver transplantation. Ceftriaxone offers certain advantages when compared with cefotaxime. First, ceftriaxone has a half-life of 6 to 10 hours,2 and therefore, can be given less frequently than cefotaxime, which has a half-life of 1 to 2 hours.3 Second, since liver transplant recipients are prone to infection of the biliary region, an antibiotic that maintains an adequate oncentration in this area is desirable. As much as 40% of parent ceftriaxone is excreted into the biliary tract,4 whereas cefotaxime is eliminated solely through the kidneys as the parent compound and as the metabolites.3 Wider pathogenic spectrum is another advantage of ceftriaxone.1,5 Since a large fraction of the dose of ceftriaxone is excreted in the bile, the functional status of the liver is expected to influence the disposition of ceftriaxone. The objective of the present study was to elucidate ceftriaxone pharmacokinetics in orthotopic liver transplant patients, and subsequently determine an optimized dosing regimen in this patient population.
MATERIALS AND METHODS
Subjects
Seven adult orthotopic liver transplant patients, five men and two women, who were receiving intravenous cefotaxime as part of their post-operative medication were randomly chosen to participate in the present study. Written informed consent was obtained from all subjects, and the study protocol was approved by the University of Pittsburgh Institutional Review Board. Patients on any known enzyme inducers or inhibitors were excluded from the study.
Study Design
Three to 5 days after transplant, a 2 gram intravenous dose of ceftriaxone was substituted for a single dose of cefotaxime. Ceftriaxone was given as an intravenous infusion over 15 minutes. Blood samples (2.5 mL) were collected before and at 7, 15, and 30 minutes and 1, 2, 3, 4, 6, 8, 10, 12, 18, and 24 hours after the start of the infusion. Blood was centrifuged at room temperature, and the plasma was separated and frozen at −70°C until assayed. Urine (24 hr) and bile output were also collected via a catheter and a T-tube, respectively. These samples were measured, aliquoted, and frozen at −70°C until assayed.
Assay
Samples were processed by a modification of the method of Patel et al.6 The sample, 250 μL of plasma, 100 μL or urine, or 100 μL of bile, was added to 0.75 mL of water; 2 mL of acetonitrile containing 5 μg/mL of medazepam, the internal standard, were added. After shaking for 10 minutes and centrifugation for 10 minutes at 1500 × g, 30 μL of the supernatant were analyzed by HPLC. The chromatographic system consisted of a 150 × 4.6 mm 5μ C18 column (Supelco, Belfonte, PA) maintained at 40°C with ultraviolet detection at 254 nm. The mobile phase consisted of acetonitrile, 1% hexadecyltrimethylammonium bromide (HTAB) in water, 1M phosphate buffer pH 7 and water (44:30:1:25). At a flow rate of 1.5 mL/min, the retention times of ceftriaxone and medazepam were 4.5 and 12 minutes, respectively (Figure 1). The standard curve was linear between 5 and 200 μg/mL and the day-to-day coefficient of variation was <15%.
Figure 1.
Representative chromatograms that were obtained from subject #6: A, blank plasma; B, plasma 10 hr after drug administration. Peaks C and IS represent drug and internal standard, respectively; estimated ceftriaxone concentration: 62 μg/mL.
Protein Binding
Five plasma samples from each patient, representing approximately every other time point, were used to determine ceftriaxone plasma protein binding by equilibrium dialysis. Plasma (400 μL) was dialyzed against an equal volume of pH 7.4 isotonic phosphate buffer at 37°C. After a 4-hour equilibration time, the buffer and plasma were removed and assayed for ceftriaxone concentrations by HPLC as described earlier. Preliminary studies indicated that equilibrium was achieved in 4 hours and that no volume shift correction was necessary. The percent of ceftriaxone bound to plasma proteins was determined by the following equation:
Data Analysis
Plasma data of total ceftriaxone were analyzed by standard noncompartmental methods.7 Data from the protein-binding study were fitted using MINSQ least-square parameter estimating program8 to the following equation9:
where β is the ratio of bound to total plasma concentration, Cu is the unbound concentration in plasma, Ka is the affinity constant of the drug-protein interaction, and nP is the capacity constant. Using parameter estimates for each subject, the unbound concentration corresponding to each time point was calculated using the following equation10:
where Kd = 1/Ka, Ctot is the total concentration in plasma.
Correlation among various pharmacokinetic parameters and biochemical indices of the patients was examined by linear regression.11
RESULTS
The characteristics of the patients who were participating in the present study are summarized in Table I. Two patients, 3 and 7, had markedly impaired renal function as determined by a serum creatinine level (Scr) >2 mg/dl. Liver function in all the patients was below normal, as determined by various biochemical indices (Table I). Table II summarizes the pharmacokinetic parameters from plasma data; urine and bile data are shown in Table III. Table IV lists the correlations between the various pharmacokinetic parameters and biochemical indices.
TABLE I.
Biochemical Profile of Liver Transplant Patients*
| Pt. # | Sex | Age (yr) |
BW (kg) |
BUN (mg%) |
SCr (mg%) |
SGOT (U/L) |
SGPT (U/L) |
Bilirubin† (mg%) |
AP (U/L) |
GGT (U/L) |
Albumin (g%) |
TP (g%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 57 | 83 | 24 | 1.1 | 270 | 434 | 0.8/1.4 | 69 | 44 | 2.0 | 4.1 |
| 2 | M | 40 | 90 | 34 | 1.4 | 265 | 240 | 5.6/7.6 | 30 | 21 | 3.7 | 6.0 |
| 3 | M | 50 | 69 | 58 | 2.7* | 1022 | 856 | 2.6/3.3 | 51 | 62 | 2.9 | 5.2 |
| 4 | F | 37 | 70 | 8 | 0.6 | 352 | 346 | 4.6/6.0 | 246 | 135 | 2.9 | 7.7 |
| 5 | M | 58 | 70 | 37 | 1.3 | 119 | 60 | 11.5/13.0 | 508 | 511 | 2.6 | 5.1 |
| 6 | M | 55 | 64 | 14 | 0.8 | 43 | 83 | 1.8/2.4 | 153 | 170 | 2.2 | 4.5 |
| 7 | F | 55 | 79 | 51 | 2.6* | 114 | 138 | 1.6/2.2 | 56 | 56 | 2.9 | 5.3 |
| Mean | 50 | 75 | 32 | 1.5 | 312 | 308 | 4.1/5.1 | 159 | 143 | 2.7 | 5.4 | |
| SD | 8 | 9 | 18 | 0.8 | 331 | 278 | 3.7/4.1 | 171 | 171 | 0.6 | 1.2 |
Normal values: BUN, 7–22 mg%; Scr, 0.5–1.4 mg%; SGOT, 12–45 U/L; SGPT, 7–40 U/L; Bilirubin, 0.1–0.4 mg%/0.3–1.3 mg%; Alk phos, 37–107 U/L; GGT, 8–69 U/L; Albumin, 3.5–4.8 g%; Total protein, 6.1–8.0 g%.
Direct/total.
TABLE II.
Pharmacokinetics of Ceftriaxone in Liver Transplant Recipients
| Patient Number |
Mean ± SD |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | Mean ± SD | Normal Subjects* | ||
| I. | Protein Binding | |||||||||
| nP, mM × 104 | 1.64 | 3.45 | 2.47 | 2.95 | 1.26 | 1.04 | 4.22 | 2.43 ± 1.19 | 4.33 ± 0.87 | |
| Ka, mM−1 × 10−4 | 5.92 | 1.74 | 5.90 | 1.76 | 1.48 | 9.83 | 0.56 | 3.89 ± 3.40 | 6.49 ± 1.15 | |
| II. | Total Drug | |||||||||
| Cmax, μg/mL | 290 | 218 | 306 | 315 | 245 | 560 | 293 | 318 ± 112 | 257 ± 17 | |
| C12, μg/mL | 61 | 84 | 90 | 33 | 48 | 54 | 117 | 70 ± 29 | 46 ± 7 | |
| C24, μg/ml | 21 | 68 | 81 | 13 | 28 | 19 | 104 | 48 ± 36 | 15 ± 4 | |
| TBC, mL/hr | 994 | 368 | 311 | 1706 | 945 | 1059 | 260 | 806 ± 526 | 1190 ± 150 | |
| mL/hr/kg | 12.0 | 4.1 | 4.5 | 24.4 | 13.5 | 16.5 | 3.3 | 11.2 ± 7.8 | ||
| MRT, hr | 12.5 | 58.8 | 44.6 | 8.5 | 25.8 | 10.7 | 48.5 | 29.9 ± 20.6 | ||
| Vss, L | 12.4 | 21.7 | 13.9 | 14.5 | 24.4 | 11.4 | 12.6 | 15.8 ± 5.1 | ||
| mL/kg | 150 | 241 | 201 | 207 | 349 | 178 | 159 | 212 ± 68 | ||
| Varea, L | 13.1 | 20.9 | 16.3 | 14.7 | 26.7 | 12.7 | 12.1 | 16.6 ± 5.3 | 10.1 ± 1.0 | |
| mL/kg | 158 | 232 | 236 | 210 | 381 | 199 | 153 | 224 ± 76 | ||
| β, hr−1 | 0.076 | 0.018 | 0.019 | 0.116 | 0.035 | 0.083 | 0.021 | 0.053 ± 0.039 | ||
| t1/2, hr | 9.2 | 39.4 | 36.3 | 6.0 | 19.6 | 8.3 | 32.4 | 13.1b | 5.8(4.7–6.8)‡ | |
| III. | Unbound Drug | |||||||||
| Cmax, μg/mL | 187 | 70 | 154 | 157 | 178 | 492 | 140 | 197 ± 136 | ||
| C12, μg/mL | 10 | 16 | 11 | 6 | 21 | 11 | 42 | 17 ± 12 | ||
| C24, μg/mL | 2 | 12 | 9 | 2 | 11 | 2 | 37 | 11 ± 12 | ||
| TBC, mL/hr | 4322 | 2318 | 3085 | 7163 | 2245 | 2980 | 794 | 3272 ± 2019 | 14940 ± 2160 | |
| mL/hr/kg | 52.1 | 25.8 | 44.7 | 102.3 | 32.1 | 46.6 | 10.0 | 44.8 ± 29.1 | ||
| MRT, hr | 6.6 | 44.9 | 25.0 | 6.2 | 21.0 | 4.7 | 40.7 | 21.3 ± 16.7 | ||
| Vss, L | 28.6 | 104.1 | 77.2 | 44.3 | 47.2 | 14.0 | 32.3 | 49.7 ± 31.0 | ||
| mL/kg | 345 | 1156 | 1119 | 634 | 674 | 218 | 409 | 651 ± 368 | ||
| Varea, L | 38.8 | 102.7 | 104.0 | 54.3 | 54.0 | 20.2 | 31.1 | 57.9 ± 33.3 | 162 ± 18 | |
| mL/kg | 467 | 1141 | 1507 | 776 | 771 | 315 | 394 | 767 ± 432 | ||
| β, hr−1 | 0.111 | 0.023 | 0.030 | 0.132 | 0.042 | 0.148 | 0.026 | 0.073 ± 0.055 | ||
| t1/2, hr | 6.2 | 30.7 | 23.4 | 5.3 | 16.7 | 4.7 | 27.2 | 9.5† | 7.6 | |
TABLE III.
Pharmacokinetics of Ceftriaxone in Liver Transplant Recipients
| Patient Number |
Mean ± SD |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | Mean ± SD | Normal Subjects* | ||
| I. | Urine | |||||||||
| % Exc, 24 hr | 83.2 | 10.9 | 36.1 | 81.9 | 31.7 | 66.1 | 20.6 | 47.2 ± 29.6 | 42 ± 10 | |
| CIR mL/hr† | 975 | 133 | 217 | 1572 | 474 | 796 | 146 | 616 ± 534 | 533 ± 128 | |
| CIR mL/hr/kg† | 11.7 | 1.5 | 3.2 | 22.5 | 6.8 | 12.4 | 1.8 | 8.6 ± 7.6 | ||
| % Exc, inf‡ | 98.1 | 36.1 | 69.8 | 92.2 | 50.1 | 75.2 | 56.2 | 68.3 ± 22.4 | ||
| II. | Bile | |||||||||
| Av Conc, μg/mL | 108 | — § | 57 | 14 | 24 | 18 | 134 | 59 ± 51 | ||
| % Exc, 24 hr | 1.21 | — | 0.55 | 0.13 | 0.25 | 0.26 | 1.74 | 0.69 ± 0.65 | ||
| CIB, mL/hr† | 14.18 | — | 3.31 | 2.49 | 3.74 | 3.13 | 12.33 | 6.53 ± 1.35 | ||
| % Exc, inf∥ | 1.43 | — | 1.06 | 0.15 | 0.40 | 0.30 | 4.75 | 1.35 ± 1.74 | 40¶ | |
TABLE IV.
Correlations Between Pharmacokinetic Parameters and the Biochemical Indices of Liver Transplant Recipients
| Bilirubin |
||||||
|---|---|---|---|---|---|---|
| Unbound | Total | Albumin | SCR | BUN | ||
| I. | Binding Parameters | |||||
| nP, mM × 104 | NS | |||||
| Ka, mM−1 × 10−4 | NS | |||||
| II. | Total Drug | |||||
| TBC, mL/hr | NS | NS | ||||
| mL/hr/kg | NS | NS | ||||
| β, hr−1 | NS | NS | ||||
| Vss, L | ↑0.003 | ↑0.001 | NS | |||
| mL/kg | ↑0.0001 | ↑0.0001 | NS | |||
| Varea, L | ↑0.001 | ↑0.001 | NS | |||
| mL/kg | ↑0.001 | ↑0.002 | NS | |||
| III. | Unbound Drug | |||||
| TBC, mL/hr | NS | NS | ||||
| mL/hr/kg | NS | NS | ||||
| β, hr−1 | NS | NS | ||||
| % Exc24 | NS | NS | ||||
| ClR, mL/hr | NS | NS | ||||
| mL/hr/kg | ↑0.05 | ↑0.02 | ||||
| % Excinf | ↑0.02 | ↑0.025 | ||||
Protein Binding
Nonlinearity in the plasma protein binding of ceftriaxone was evident throughout the concentration range attained in the present study; over the concentration range of the samples measured, 13 to 243 μg/mL, the unbound fraction in plasma ranged from 0.05 to 0.56. Capacity constant (nP) and affinity constant (Ka) showed considerable variability among subjects (Table II). As shown in Table II, binding parameters are markedly different from those calculated for normal subjects.4 No significant correlation was found between binding parameters and albumin concentration (Table IV).
Plasma
Figures 2 and 3 show the plasma concentration–time profiles of total and unbound ceftriaxone in two patients. The various pharmacokinetic parameters for total and unbound ceftriaxone are shown in Table II. Total body clearance (TBC) of total ceftriaxone was 11.2 ± 7.8 mL/hr/kg. TBC for unbound drug was 44.8 ± 29.1 mL/hr/kg. No significant correlation was found between TBC of either total or unbound drug and bilirubin levels (Table IV); only a slight trend of a negative relationship (P < .07) was found between the clearance of unbound drug and total bilirubin. The volume of distribution (Varea) for total and unbound drug was 224 ± 76 mL/kg and 767 ± 432 mL/kg, respectively. The steady-state volume of distribution (Vss) was 212 ± 68 mL/kg for total ceftriaxone and 651 ± 368 mL/kg for the unbound drug in plasma. The volumes of distribution for total, but not unbound, ceftriaxone showed a significant positive correlation with bilirubin levels (Table IV). Terminal disposition rate constants (Table II) were 0.053 ± 0.039 hour−1 and 0.073 ± 0.055 hour−1 for total and unbound ceftriaxone, respectively, and did not correlate significantly with bilirubin levels (Table IV). The terminal disposition plasma half-life was 21.6 ± 14.3 hour for total and 16.3 ± 11.1 hour for unbound ceftriaxone. The mean residence time (MRT) for total and unbound drug was 29.9 ± 20.6 hour and 21.3 ± 16.7 hour, respectively.
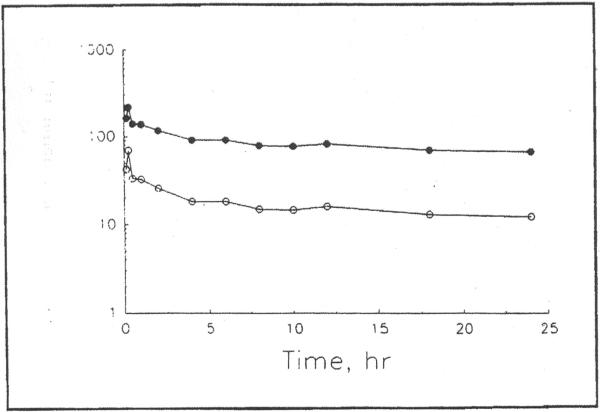
Figure 2.
Plasma concentration–time profile of total (●) and estimated unbound (○) ceftriaxone in a liver transplant recipient (#2) after a 2 g dose given as a 15-min intravenous infusion.
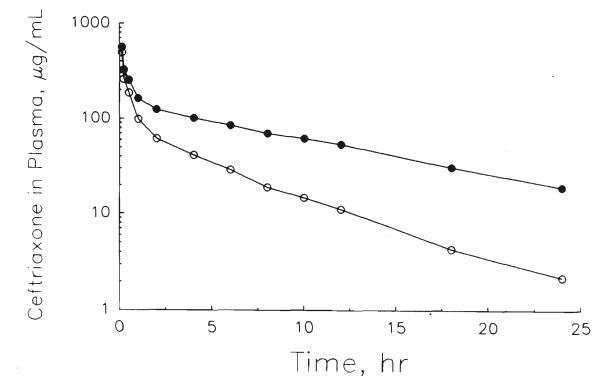
Figure 3.
Plasma concentration–time profile of total (●) and estimated unbound (○) ceftriaxone in a liver transplant recipient (#6) after a 2 g dose given as a 15-min intravenous infusion.
Urine and Bile
The percentage of dose that was excreted in urine over 24 hours (Table III) was 47.2 ± 29.6% and did not correlate significantly with Scr or BUN (Table IV). Estimated percent that was excreted in urine at time infinity was 68.3 ± 22.4% and correlated inversely with Scr and BUN (Table IV). Renal clearance, calculated from 24-hour urine and plasma data, was 8.6 ± 7.6 mL/hr/kg and correlated inversely with both Scr and BUN.
The percentage of the dose excreted in bile over 24 hour was 0.69%±0.65 (Table III) and did not correlate significantly with bilirubin levels (Table IV).
Various pharmacokinetic parameters that were calculated in the present study were compared with corresponding literature values in healthy volunteers for total and unbound ceftriaxone (Tables II and III). For both total and unbound drug, TBC was lower in the liver transplant patients; the difference is more pronounced with respect to unbound as compared with total drug. Varea for total ceftriaxone was considerably greater, whereas that of unbound drug was smaller in the liver transplant recipients compared with normal subjects. Plasma ceftriaxone concentrations after 12 hours (C12) and 24 hours (C24) were considerably higher in this patient group as compared with normal subjects who were receiving the same dose of the drug.
DISCUSSION
Ceftriaxone offers certain practical advantages such as a longer half-life and higher biliary excretion as compared with cefotaxime. The present study determined whether ceftriaxone disposition in liver transplant recipients during the early post-operative period is different from its disposition in healthy subjects, and determined whether a 2 g/day dose will provide plasma ceftriaxone concentrations above the reported MIC.
Ceftriaxone exhibits nonlinear kinetics and comparison of the parameters that were calculated in the present study for total drug with literature values in normal subjects was therefore confined to data from subjects who were receiving the same dose, i.e., 2 g. Since binding is the only nonlinear process in ceftriaxone disposition,4,8,12,13 the unbound drug is expected to behave linearly, and comparison was made among various reports regardless of the dose.
Most of the pharmacokinetic parameters that were calculated in the present study were notably different from literature values for normal subjects and exhibited much greater variability (Tables II and III). Such variability in kinetics may be related to the patient population that was studied. Even though Cmax observed in this study was comparable to that observed in normal subjects, much higher concentration was maintained at 12 and 24 hours after drug administration (Table II). In all subjects, C24 was higher than the MIC50 of most susceptible organisms.14 The mean TBC of unbound ceftriaxone that was obtained in this study is approximately one fifth of the value reported for normal subjects (Table II). Such reduction could be explained on the basis of compromised liver function in the early post-operative period. Injury to the organ that was incurred by cold ischemia and warm reperfusion, resulting in an impairment of drug uptake by hepatocytes and of biliary excretion, may account for the reduction in the unbound clearance of ceftriaxone. In addition, four of the seven patients who were studied had a bilirubin concentration of greater than 2 mg/dL. Minor changes in the pharmacokinetic parameters of total ceftriaxone have been observed in patients with liver disease15 and in patients with cholecystectomy.16
The volume of distribution of total drug is greater in patients than in normal subjects, whereas the opposite is observed with unbound volume of distribution (Table II). The larger volume of distribution is the result of decreased plasma protein binding due to decreased albumin concentration and decreased affinity of ceftriaxone for plasma proteins. In addition, the positive correlation that was obtained between the volume of distribution of total ceftriaxone and bilirubin, may indicate competition between with ceftriaxone and bilirubin for albumin binding sites. The reduction in unbound volume of distribution may be a reflection of the decrease in the uptake of ceftriaxone by body tissues.
The half-life of ceftriaxone was significantly increased in liver transplant patients. It was not possible to account for the dose that was administered by urinary and biliary excretion in 24 hours. The percentage of dose that was excreted in urine over 24 hours did not differ from literature value for normal subjects who were receiving the same dose, whereas the percent that was excreted in bile was greatly reduced; however, such a comparison may be misleading because in normal subjects 24-hour urinary or biliary excretion approximates the total amount eliminated by the respective route, whereas in patients, in whom the terminal disposition half-life of ceftriaxone is markedly longer, the 24-hour urinary or biliary excretion is only a fraction of the total amount that is eliminated via each route. A better comparison may be made between the estimated percent that was excreted in urine or bile at time infinity in patients to the 24-hour urinary or biliary excretion in normal subjects (Table III).
It could be inferred from the large fraction that was eliminated by urinary excretion and the small fraction that was eliminated in bile that the kidneys contribute more to the elimination of ceftriaxone in liver transplant recipients than in normal subjects. Although total 24-hour biliary recovery of ceftriaxone (Table III) was markedly lower than that reported in normal subjects,2 the pooled biliary concentration in all subjects (Table V) was higher than the MIC50 of most of the susceptible organisms.14
In conclusion, the disposition of ceftriaxone in liver transplant recipients in the early post-operative period is considerably different from normal subjects. Clearance, binding, volume of distribution, and half-life are different from normal subjects, and lower doses of ceftriaxone may be sufficient to prevent infection. Due to high variability in the clearance, a minimum dose of 2 g/day is expected to maintain adequate antibiotic concentrations in all patients.
REFERENCES
- 1.Angehrn P, Probst PJ, Reiner R, Then RL. Ro 13-9904, a long-acting broad-spectrum cephalosporin: In vitro and in vivo studies. Antimicrob Agents Chemother. 1980;18:913–921. doi: 10.1128/aac.18.6.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Harding SM. Pharmacokinetics of the third-generation cephalosporins. Am J Med. 1985;79(suppl 2A):21–24. doi: 10.1016/0002-9343(85)90256-6. [DOI] [PubMed] [Google Scholar]
- 3.Esmieu F, Guibert J, Rosenkilde HC, Ho I, Le Go A. Pharmacokinetics of cefotaxime in normal human volunteers. J Antimicrob Chemother. 1980;6(suppl A):83–92. doi: 10.1093/jac/6.suppl_a.83. [DOI] [PubMed] [Google Scholar]
- 4.Stoeckel K, McNamara PJ, Brandt R, Plozza-Nottebrock H, Ziegler WH. Effects of concentration-dependent plasma protein binding on ceftriaxone kinetics. Clin Pharmacol Ther. 1981;29:650–7. doi: 10.1038/clpt.1981.90. [DOI] [PubMed] [Google Scholar]
- 5.Neu HC. The new beta–lactamase-stable cephalosporins. Ann Intern Med. 1982;97:408–419. doi: 10.7326/0003-4819-97-3-408. [DOI] [PubMed] [Google Scholar]
- 6.Patel IH, Chen S, Parsonnet M, Hackman MR, Brooks MA, Konikoff J, Kaplan SA. Pharmacokinetics of ceftriaxone in humans. Antimicrob Agents Chemother. 1981;20:634–641. doi: 10.1128/aac.20.5.634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gibaldi M, Perrier D. Pharmacokinetics. Second edition Marcel Dekker; New York: 1982. pp. 409–417. [Google Scholar]
- 8.MINSQ . Least Square Parameter Estimation. MicroMath Scientific Software; Salt Lake City: 1988. [Google Scholar]
- 9.Shargel L, Yu ABC. Applied Biopharmaceutics and Pharmacokinetics. Appleton-Century-Crofts; Norwalk: 1985. pp. 204–7. [Google Scholar]
- 10.Coffey JJ, Bullock FJ, Schoenemann PT. Numerical solution of nonlinear pharmacokinetic equations: Effect of plasma protein binding on drug distribution and elimination. J Pharm Sci. 1971;60:1623–8. doi: 10.1002/jps.2600601106. [DOI] [PubMed] [Google Scholar]
- 11.Rosner B. Fundamentals of Biostatistics. Duxbury; Boston: 1982. pp. 344–366. [Google Scholar]
- 12.Popick AC, Crouthamel WG, Bekersky I. Plasma protein binding of ceftriaxone. Xenohiotica. 1987;17:1139–1145. doi: 10.3109/00498258709167406. [DOI] [PubMed] [Google Scholar]
- 13.Hamilton RA, Kowalsky SF, McCormick EM, Echols RM. Protein binding of ceftriaxone, cefoperazone, and ceftizoxime. Clin Pharm. 1987;6:567–569. [PubMed] [Google Scholar]
- 14.Ghosen V, Chamali R, Bar-Moshe O, Stenier P. Clinical study of Rocephin®, 1 3rd generation cephalosporin, in various septicaemias. Chemotherapy. 1981;27(Suppl 1):100–103. doi: 10.1159/000238036. [DOI] [PubMed] [Google Scholar]
- 15.Stoeckel K, Tuerk H, Trueb V, McNamara PJ. Single-dose ceftriaxone kinetics in liver insufficiency. Clin Pharmacol Ther. 1984;36:500–509. doi: 10.1038/clpt.1984.210. [DOI] [PubMed] [Google Scholar]
- 16.Hayton WL, Schandlik R, Stoeckel K. Biliary excretion and pharmacokinetics of ceftriaxone after cholecystectomy. Eur J Ciin Pharmacol. 1986;30:445–451. doi: 10.1007/BF00607958. [DOI] [PubMed] [Google Scholar]