Abstract
Metastatic renal cell carcinoma (RCC) presenting with multiple deposits in the head and neck region is unusual. It is not uncommon for a RCC to metastasise to a distant site after years of a tumour-free period, but most of it would be expected to have a single site of deposit. We report a rare case of a patient who had a nephrectomy 10 years earlier for RCC and presented with tumours in the frontal sinus and posterior pharyngeal wall. Radiological imaging and histology confirmed metastatic RCC at both sites.
Keywords: cancer of the head and neck, diagnostic imaging, neoplasm metastases, oncology, renal cell carcinoma, signs and symptoms
Introduction
Renal cell carcinoma (RCC) is the most frequent urological malignancy in adults and has a male preponderance. It accounts for approximately 3% of adult malignancies and 90%–95% of neoplasms arising from the kidney. Metastases have been reported to develop 17 years or more after the primary lesion is removed (1). RCC usually metastasises to lungs, bones, and regional lymph nodes, but very rarely to the head and neck region (2). We report a very rare presentation of a male patient, previously diagnosed with RCC, with metastasis in the head and neck region.
Case Report
This case was of an 80-year-old man diagnosed with RCC at stage T3N0M0 in 1998. He underwent a left radical nephrectomy and was well during the 10-year follow-up, with no evidence of recurrence. In July 2008, he presented with a swelling at the left frontal region, which had persisted for 9 months and was progressively increasing in size and associated with left proptosis. However, there was no reduced vision, diplopia, persistent headache, nasal obstruction, or epistaxis. In addition, he complained of a foreign-body sensation in the throat for the previous month, but without odynophagia, dysphagia, or hoarseness. Physical examination revealed a large left frontal mass, measuring 6 x 5 cm, soft to firm in consistency, non-tender, and with normal overlying skin. It caused displacement of the left eye inferolaterally. Nasal endoscopy showed a mass at the left frontal recess pushing the middle turbinate medially and uncinate process laterally. Another mass was found at the posterior pharyngeal wall. A biopsy was taken from the nasal mass during the clinic visit. This resulted in profuse epistaxis requiring a nasal packing. The histological report demonstrated clear cell carcinoma showing malignant cells that exhibited large pleomorphic nuclei with conspicuous nucleoli and abundant, clear cytoplasm. Immunohistochemistry showed that the tumour cells expressed cytokeratin (CK), CD10, EMA, and vimentin. Magnetic resonance imaging (MRI) of the brain showed a large frontonasal tumour involving the ethmoid sinus and both frontal sinuses, with intraorbital and intracranial extension (Figure 1). Computed tomography (CT) of the neck showed a contrast-enhanced 1 x 1 cm mass at the posterior pharyngeal wall (Figure 2). The patient underwent an endoscopic-assisted craniofacial resection of the tumour with pre-operative embolisation because of the high vascularity of the tumour on imaging. Intraoperative findings revealed a large, vascular, soft tumour involving the left frontal and both frontal sinuses. It had eroded the lamina papyracea on both sides and invaded the dura. Because of the extensive involvement of the surrounding tissues, a tumour-debulking surgery was performed. The tumour was removed piecemeal from its posterior extension via craniofacial resection to its inferior extent via endoscopic approach. Orbital exenteration was not performed because the patient did not consent to it. A direct laryngoscopy showed a vascular tumour measuring 1.0 x 1.5 cm with a pedicle arising from the posterior pharyngeal wall. It was excised completely. Post-operative recovery was uneventful, and the patient was discharged on the seventh post-operative day. Histopathological examination from the frontonasal and the posterior pharyngeal tumours confirmed the same histology (Figure 3) and immunophenotype (Figure 4) as in the earlier biopsy. On followup, there was residual tumour at the left frontal recess. Surgery was recommended for excision of the residual tumour. However, the patient refused further treatment.
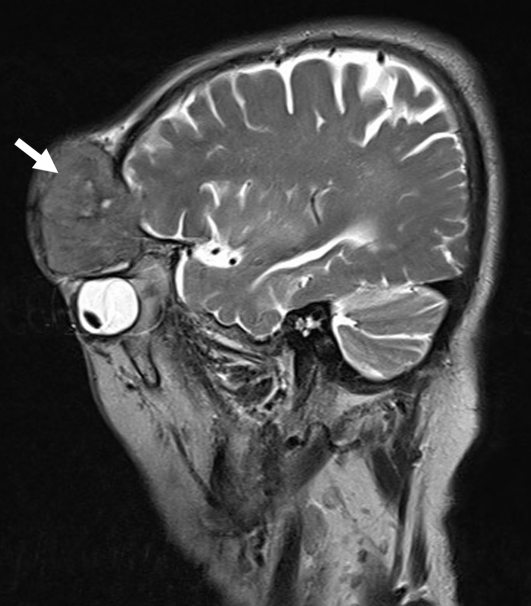
Figure 1:
Brain T2-weighted magnetic resonance image (sagittal view), showing the frontonasal tumour (arrow) with intraorbital and intracranial extension.
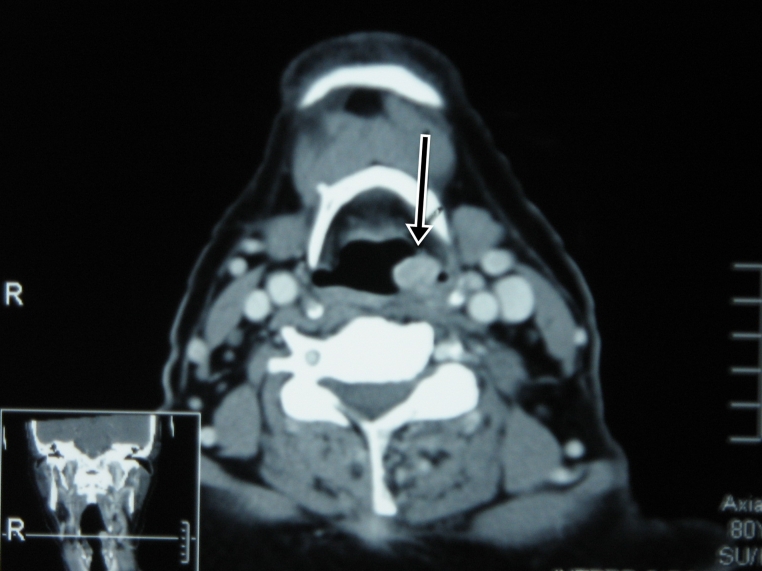
Figure 2:
Neck computed tomogram image showing a laryngeal mass at the posterior pharyngeal wall (arrow).
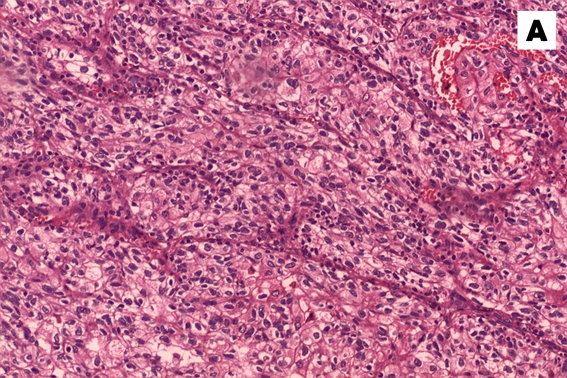
Figure 3:
Haematoxylin and eosin stain of the tumour specimen. (A) A low-power view (10x magnification) showed groups of neoplastic cells separated by fibrous septa with intervening blood vessels. (B) The cell morphology is better appreciated in the high-power view (40x magnification), which clearly revealed the neoplastic features of the cells with pleomorphic nucleus, some with conspicuous nucleoli and abundant, clear to eosinophilic cytoplasm.
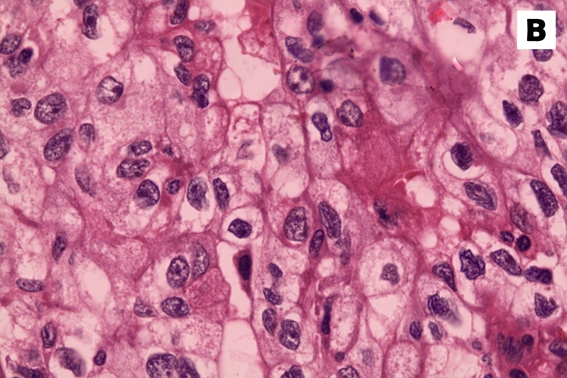
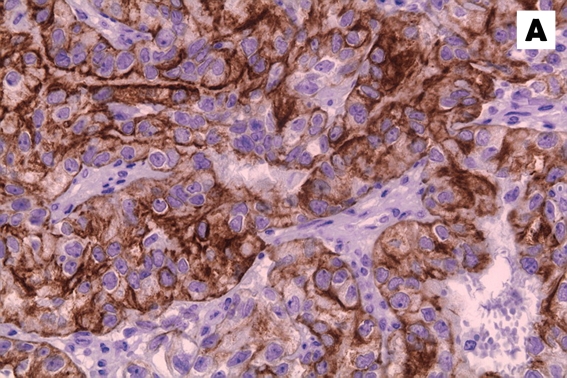
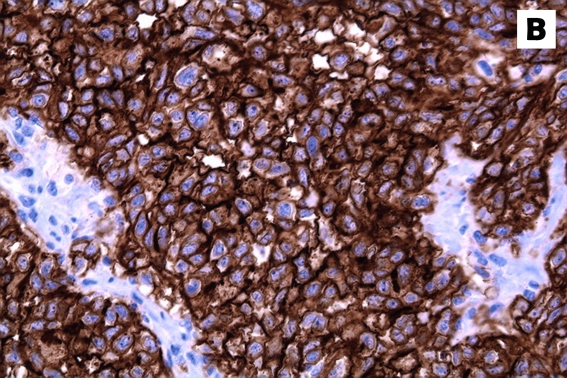
Figure 4:
Immunohistochemical stains of the tumour specimen showed that the neoplastic cells were strongly positive for (A) cytokeratin and (B) CD10.
Discussion
In patients with RCC, tumour stage, regional lymph node status, tumour size, nuclear grade, and histological tumour necrosis show statistically significant associations with metastasis (3). RCC has frequently been reported to metastasise to the lung, liver and bone, whereas metastases to the head and neck region constitute only about 15% of cases (2). Common sites for metastasis in the head and neck region include paranasal sinuses, thyroid, larynx, mandible, and parotid glands (1), and they are usually present at a single site. Previously resected RCC that metastasises to multiple sites in the head and neck region is very rare. Extensive searches of the medical literature have, so far, not shown any reported cases of multiple metastases in the head and neck region. Pritchyk et al. (4) reported 4 cases of metastatic RCC, all with single metastatic sites. In addition, Gottlieb and Roland (1) reported cases with single metastatic sites. Other studies have also reported single sites of metastasis in the head and neck region (5,6).
The clinical behaviour of metastatic RCC is unpredictable, and the signs and symptoms depend on the affected organ and the presence of local invasion. Metastatic deposits from RCC are vascular in nature and often bleed. The vascular stroma of metastatic RCC is responsible for the fact that the most common symptom of sinonasal lesions is epistaxis in 70% of cases (7). An office-based biopsy of the lesion could result in uncontrolled haemorrhage, as demonstrated in this patient. A contrast CT scan of the paranasal sinuses would have shown the highly vascular nature of the lesion. Once the vascular nature of the lesion is known, a biopsy should be performed in the operating room.
Clear cell carcinoma is the most common histological variant of RCC. The clear cells are rounded or polygonal with abundant cytoplasm, which contains cholesterol, cholesterol esters, phospholipids, and glycogen. These are dissolved during routine histological processing, creating a clear cytoplasm surrounded by a distinct cell membrane. Histopathological examination commonly shows a network of small, thin-walled blood vessels interlacing between the tumour cells. Immunohistochemical staining typically shows that the tumour is positive for CK, CD10, EMA, and vimentin. Other tumours may also contain clear cells; these include acinic cell carcinoma, mucoepidermoid carcinoma, odontogenic clear cell carcinoma, and metastatic clear cell carcinoma of the thyroid. Histochemical and morphological analysis is useful in the differential diagnosis of these lesions and the clear cell carcinoma of the head and neck.
Treatment should be individualised according to the tumour location and general health of the patient. Our patient was subjected to craniofacial surgery mainly to debulk the tumour mass and relieve compression to the brain and orbit. Historically, the role of surgery in metastatic RCC has been to diagnose and debulk the disease. A review of the literature revealed that excision of solitary metastatic lesions of RCC following nephrectomy results in 41% survival at 2 years and 13% survival at 5 years, regardless of the time interval between nephrectomy and diagnosis of the metastatic lesion (2). For small, localised disease at the nasal and paranasal region, endoscopic resection is recommended to decrease post-operative morbidity. However, for more extensive disease with local spread, such as intracranial or intraorbital extension, we believe that endoscopic-assisted craniofacial debulking is the treatment of choice to improve quality of life. This, however, is technically challenging and may result in an inadequate resection for palliation of symptoms. Therefore, an open approach is more appropriate. Regardless of the approach, the goal of therapy should be an adequate local resection that allows for appropriate short-term palliation of symptoms, despite the poor long-term prognosis of the disease (4). This offers improved quality of life, may provide a chance for a cure for the head and neck metastasis, and is warranted based on the associated morbidity that may occur if the lesion is left untreated.
Other forms of treatment have been advocated for metastatic RCC. Immunotherapy with interferon (IFN)-α and interleukin (IL)-2 has been the mainstay of treatment for people with advanced and metastatic RCC. However, this treatment is frequently associated with unpleasant side effects and confers only modest benefits. Recent advances in understanding the biology and genetics of RCC have led to several novel, targeted approaches to treat metastatic RCC, with higher response rates. Treatment with sunitinib (oral multi-targeted tyrosine kinase inhibitor), sorafenib, bevacizumab/erlotinib (recombinant humanised monoclonal antibody), and CCI-779 (temsirolimus) have shown promising results in terms of progression-free survival (8). Previously, hormonal treatment and standard chemotherapies have been described, but they are generally not considered effective due to high levels of drug resistance (9).
In conclusion, metastasis of RCC to multiple sites in the head and neck region is rare. Awareness of other metastatic sites in this region is required, and careful evaluation should be done to identify these metastatic deposits. Although surgery may not be adequate for tumour clearance, it is crucial to palliate symptoms and to reduce the morbidity associated with it.
Footnotes
Authors’ Contributions
Collection and assembly of data: AII, SHMP
Drafting of the article: AII
Critical revision of the article: NM, GBS
Final approval of the article: GBS
References
- 1.Gottlieb MD, Roland JT., Jr Paradoxical spread of renal cell carcinoma to the head and neck. Laryngoscope. 1998;108(9):1301–1305. doi: 10.1097/00005537-199809000-00007. [DOI] [PubMed] [Google Scholar]
- 2.Som PM, Norton KI, Shugar JM, Reede DL, Norton L, Biller HF, et al. Metastatic hypernephroma to the head and neck. AJNR Am J Neuroradiol. 1987;8(6):1103–1106. [PMC free article] [PubMed] [Google Scholar]
- 3.Leibovich BC, Blute ML, Cheville JC, Lohse CM, Frank I, Kwon ED, et al. Prediction of progression after radical nephrectomy for patients with clear cell renal cell carcinoma: A stratification tool for prospective clinical trials. Cancer. 2003;97(7):1663–1671. doi: 10.1002/cncr.11234. [DOI] [PubMed] [Google Scholar]
- 4.Som PM, Norton KI, Shugar JM, Reede DL, Norton L, Biller HF, et al. Metastatic hypernephroma to the head and neck. AJNR Am J Neuroradiol. 1987;8(6):1103–1106. [PMC free article] [PubMed] [Google Scholar]
- 5.Pritchyk KM, Schiff BA, Newkirk KA, Krowiak E, Deeb ZE. Metastatic renal cell carcinoma to the head and neck. Laryngoscope. 2002;112(9):1598–1602. doi: 10.1097/00005537-200209000-00012. [DOI] [PubMed] [Google Scholar]
- 6.Langille G, Taylor SM, Bullock MJ. Metastatic renal cell carcinoma to the head and neck: Summary of 21 cases. J Otolaryngol Head Neck Surg. 2008;37(4):515–521. [PubMed] [Google Scholar]
- 7.Stodulski D, Stankiewicz C, Skorek A. Renal cell carcinoma metastases to the head and neck. Otolaryngol Pol. 2006;60(6):893–899. [PubMed] [Google Scholar]
- 8.Simo R, Sykes AJ, Hargreaves SP, Axon PR, Birzgalis AR, Slevin NJ, et al. Metastatic renal cell carcinoma to the nose and paranasal sinuses. Head Neck. 2000;22(7):722–727. doi: 10.1002/1097-0347(200010)22:7<722::aid-hed13>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 9.Thompson Coon JS, Liu Z, Hoyle M, Rogers G, Green C, Moxham T, et al. Sunitinib and bevacizumab for first-line treatment of metastatic renal cell carcinoma: A systematic review and indirect comparison of clinical effectiveness. Br J Cancer. 2009;101(2):238–243. doi: 10.1038/sj.bjc.6605167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Motzer RJ, Bander NH, Nanus DM. Renal-cell carcinoma. N Engl J Med. 1996;335(12):865–875. doi: 10.1056/NEJM199609193351207. [DOI] [PubMed] [Google Scholar]