Figure 6.
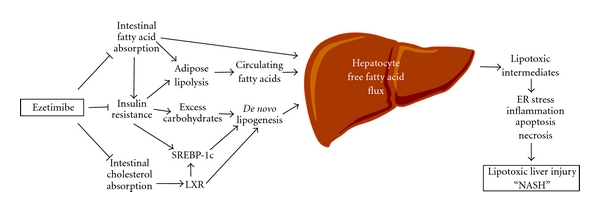
Potential therapeutic effects of ezetimibe on nonalcoholic fatty liver disease and steatohepatitis (NAFLD and NASH). On the basis of the lipotoxicity model of NAFLD and NASH [51], it has been proposed that metabolites of unesterified fatty acids may induce lipotoxic hepatocellular injury manifested as ER stress, inflammation, apoptosis, necrosis, and dysmorphic features such as ballooning and Mallory-Denk body formation. The generation of lipotoxic metabolites of fatty acids often takes place in parallel with the accumulation of triglyceride droplets (steatosis) in the liver. A high-fat diet often causes insulin resistance, a state that is associated with hyperinsulinemia and hyperglycemia. Because insulin resistance promotes an excessive flow of fatty acids from adipose tissue and also impairs peripheral glucose disposal, these alterations increase the need for fatty acid disposal in the liver through oxidative pathways and through the formation of triglyceride which is then either stored temporarily as lipid droplets or secreted as VLDL. Furthermore, elevated blood insulin and glucose activate transcription factors SREBP-1c to increase hepatic lipogenic gene expression. In addition, intestinal cholesterol absorption promotes hepatic lipogenesis via cholesterol-dependent activation of LXR. Ezetimibe treatment could block (i) intestinal fatty acid absorption, which could reduce a delivery of fatty acids from the gut to the adipose tissue through the chylomicron pathway; (ii) diet-induced insulin resistance in part by reducing intestinal fatty acid absorption; (iii) cholesterol-driven lipogenesis by inhibiting intestinal cholesterol absorption, which together may substantially reduce the burden of fatty acids on the liver. ER: endoplasmic reticulum; LXR: liver X receptor; SREBP-1c: sterol regulatory element-binding protein-1c.
