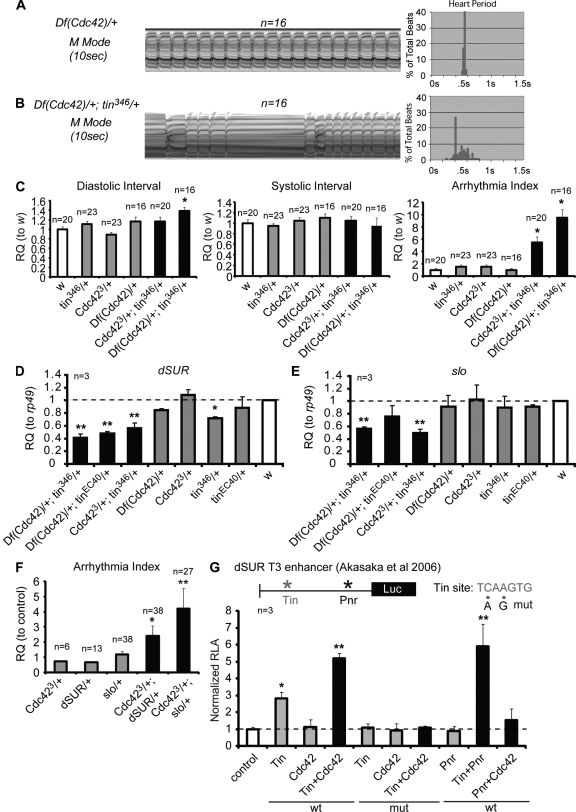
Figure 3.
Cdc42 and tinman interact to maintain normal cardiac contraction. (A and B) Representative M-mode traces showing arrhythmic heart contractions in Df(Cdc42)/+;tin346/+ (B), compared with single heterozygotes Df(Cdc42)/+ (A). (A′–B′) Heart period histograms. (C) Statistical analysis of heart contraction in controls (w, tin346/+, Cdc423/+, Df(Cdc42)/+) and Cdc423/+;tin346/+ and Df(Cdc42)/+;tin346/+ flies, shown as relative quantification (RQ). Error bars represent SEM; *, P < 0.05 (one-way ANOVA). (D and E) RQ of dSUR (A) and slowpoke (B) mRNA in 1-wk-old adult hearts (normalized to rp49, ribosomal protein 49) relative to control (w). Double heterozygotes Df(Cdc42)/+;tin346/+, Df(Cdc42)/+;tinEC40/+, and Cdc423/+;tin346/+ showed lower expression than tinman or Cdc42 heterozygotes, or w controls. (F) Functional analyses of Cdc42,dSUR and Cdc42,slo double heterozygotes showed a significant increase in the arrhythmia index. (G) Luciferase assay using the dSUR T3 enhancer (Akasaka et al., 2006). Note the increased relative luciferase activity (RLA) by transfection of Tin was further enhanced by cotransfection with Cdc42 or Pnr, but not when Pnr and Cdc42 were cotransfected. Mutation of the Tin site abolished the responsiveness. Experiments were repeated three times. Error bars represent SEM. Unpaired Student’s t test: *, P < 0.05; **, P < 0.01.