Abstract
Chemokine receptor 5 (CCR5) binds macrophage inflammatory protein 1α (MIP-1α), MIP-1β, RANTES, and members of the monocyte chemotactic protein family and is also a receptor for human immunodeficiency virus (HIV). CCR5 ligands can suppress HIV-1 entry into cells. In humans, homozygous mutations of the ccr5 gene confer resistance to HIV-1 infection. The role of CCR5 in defense against microbial infection is unclear. In this study we examined the innate and adaptive immune responses of CCR5-deficient mice to the intracellular bacterial pathogen Listeria monocytogenes. We found that migration of monocytic cells, formation of L. monocytogenes-containing lesions, and bacterial clearance occurred normally in the spleens and livers of CCR5-deficient animals. Activation of macrophages and dendritic cells during the first 3 days postinfection was normal in the absence of CCR5, as demonstrated by intact expression of inducible nitric oxide synthase (iNOS) and production of the cytokines tumor necrosis factor alpha, gamma interferon, and interleukin-12. Priming of L. monocytogenes-specific CD8 T cells also occured independently of CCR5 expression. Previously immunized, CCR5-deficient animals mounted normal secondary CD8 T-cell responses and cleared bacteria from infected organs similarly to wild-type controls, suggesting that CCR5 is dispensable for migration and activation of memory CD8 T cells. Our data indicate that CCR5-mediated chemotaxis is not required for defense against infection with L. monocytogenes.
Chemokines and chemokine receptors play an important role during inflammatory responses by governing patterns of cellular migration. Chemokine receptor 5 (CCR5) belongs to a family of G-protein-coupled receptors and is expressed on a variety of hemopoietic cell subsets crucial for the development of innate and adaptive immune responses, such as macrophages, dendritic cells (DCs), and T cells, as well as on the cells of nonhematopoietic origin (23, 31). CCR5 binds several chemokines including macrophage inflammatory protein 1α (MIP-1α), MIP-1β, RANTES (8, 28, 32), and monocyte chemotactic protein 2 (MCP-2) (17, 29).
In the mid-1990s, CCR5 was identified as a major coreceptor for human immunodeficiency virus type 1 (HIV-1), and it was subsequently demonstrated that individuals carrying the homozygous deletion of the ccr5 gene were highly resistant to infection with HIV-1 (9, 39). However, it is not clear which role, if any, CCR5 plays during normal immune responses to pathogens. Humans homozygous for the ccr5 deletion do not appear to have increased susceptibility to pathogens, suggesting that the role of this receptor in immune defense against infections might be limited (22, 33). CCR5-deficient mice were generated and have been shown to be more susceptible to the encapsulated yeast Cryptococcus neoformans (19) and to have increased susceptibility to Toxoplasma gondii (1) compared to wild-type mice. Zhou et al. demonstrated that CCR5-deficient mice display slightly reduced efficiency in clearing Listeria monocytogenes from the liver and are less susceptible to lipopolysaccharide-induced endotoxemia (44). CCR5 expression on memory phenotype CD4 and CD8 T cells has been documented, suggesting that this receptor might be utilized during the recruitment of memory T cells to the sites of inflammation in recall immune responses (31).
L. monocytogenes is a gram-positive bacterium that escapes into the cytoplasm of infected cells by secreting the pore-forming protein listeriolysin O (4). Murine listeriosis is an excellent model to study innate and adaptive immune responses to intracellular bacterial pathogens. In mice, various components of nonspecific immunity, such as neutrophils, natural killer (NK) cells, γδ T cells and CR3-positive myelomonocytic cells, are crucial for the initial control of infection (13). Secretion of the cytokines tumor necrosis factor alpha (TNF-α), interleukin-12 (IL-12), and gamma interferon (IFN-γ) and production of reactive nitrogen intermediates by inducible nitric oxide synthase (iNOS) are essential for the clearance of primary L. monocytogenes infection in vivo (5, 26, 27, 37, 42). Recently, we identified the subset of DCs that is recruited to L. monocytogenes-infected spleens in a CCR2-dependent manner and is the major source of TNF and iNOS during infection with L. monocytogenes (35). Activation of innate immunity during L. monocytogenes infection precedes the induction of adaptive immune responses composed of both CD4 and CD8 T-cell compartments. Adaptive immunity is required for complete clearance of bacteria from infected organs (18). Antigen-specific CD8 T-cell responses to Listeria have been studied extensively and are crucial for the clearance of the secondary infection (43).
In the present study, we dissected the role of CCR5 during both innate and T-cell-mediated antilisterial immune responses. We demonstrate that migration of macrophages and DCs and formation of bacterium-containing lesions occurred normally in the absence of CCR5. Activation of macrophages and DCs was also intact, as demonstrated by the induction of iNOS and secretion of TNF-α, IL-12, and IFN-γ at levels comparable to those observed in wild-type animals. Antigen-specific memory CCR5−/− CD8 T cells mediated protection during the rechallenge with L. monocytogenes and were present in both lymphoid (spleen) and nonlymphoid (liver) compartments. Our data indicate that CCR5 is dispensable for both innate and adaptive immune responses to L. monocytogenes, despite its abundant expression in the infected tissues.
MATERIALS AND METHODS
Mice and infections.
C57BL/6, CCR5−/−, C57BL/6-H2-Kd, and CCR5−/−-H2-Kd mice were bred at Memorial Sloan-Kettering Research Animal Resource Center. Generation of CCR5−/− mice was previously described (21). For most studies, CCR5−/− mice were backcrossed eight generations onto the C57BL/6 background, while for T-cell studies, mice were crossed an additional two generations onto C57BL/6-H2-Kd mice (20). For primary infections, mice were infected intravenously with 2,000 cells of L. monocytogenes strain 10403S (provided by Daniel Portnoy, University of California, Berkeley), and for secondary infections, mice were infected intravenously with 100,000 L. monocytogenes cells. At the indicated times postinfection, spleens were harvested and dissociated in phosphate-buffered saline containing 0.05% Triton X-100 and bacteria CFUs were determined by plating on brain heart infusion agar plates.
Histology.
Spleens and livers were harvested from mice infected for 3 days with L. monocytogenes, and frozen 5-μm sections were prepared. The sections were acetone fixed and incubated with goat anti-CCR5 (Santa Cruz Biotechnology, Santa Cruz, Calif.), rat anti-CD169 (SEROTEC), rat anti-Mac-3 (BD Pharmingen, San Diego, Calif.), Difco Listeria O polyserum (Fisher), and rat anti-DEC-205 and goat anti-iNOS (Santa Cruz) antibodies. Staining for L. monocytogenes was developed with anti-rabbit immunoglobulin G (IgG)-fluorescein isothiocyanate (FITC) (Jackson ImmunoResearch Laboratories, West Grove, Pa.), and staining for CCR5, CD169, DEC-205, Mac-3, and iNOS was developed with anti-IgG-biotin followed by the ABC-AP kit (Vector Laboratories, Burlingame, Calif.). Imaging of tissue sections was performed on a Zeiss Axioplan 2 microscope using Openlab software (Improvision, Lexington, Mass.).
Flow cytometry.
The following antibodies were purchased from BD Pharmingen: anti-CD11b-PerCP (M1/70), anti-CD11c-PE (HL3), anti-Mac-3-FITC (M3/84), anti-CD8α-PerCP (53-6.7), and anti-CD62L-FITC (MEL-14). Goat anti-iNOS antibody (M-19) was purchased from Santa Cruz, and anti-goat IgG-FITC was purchased from Jackson ImmunoResearch Laboratories. Phycoerythrin-conjugated streptavidin H2-Kd tetramers complexed with L. monocytogenes-derived LLO91-99 peptide for detection of antigen-specific CD8 T cells were generated as previously described (6). For DC analysis, a large gate was drawn on the live lymphocyte/monocyte population. For T-cell analysis, a gate was drawn to include lymphocyte population and cells were further gated on CD62L and CD8α. Intracellular staining was performed by staining cells for cell surface markers, fixing in 2% paraformaldehyde, permeabilizing with Perm/Wash buffer (BD Pharmingen), and incubating with anti-IFN-γ-FITC and anti-TNF-α-allophycocyanin antibodies.
ELISA.
Spleens were harvested at the indicated times postinfection, dissociated in ice-cold PBS containing 0.01% Triton X-100, and centrifuged at 10,000 × g. Murine IL-12, IFN-γ, and TNF-α in the supernatants were quantified by sandwich enzyme-linked immunosorbent assay (ELISA) using OptEIA kits from Pharmingen.
RESULTS
Normal clearance of L. monocytogenes in the organs of CCR5-deficient mice.
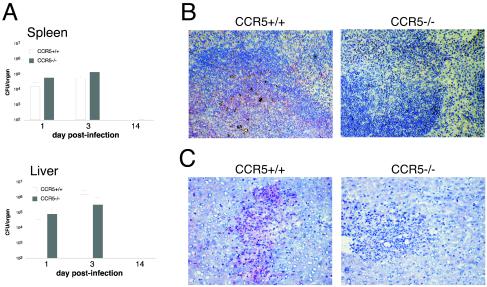
We examined early bacterial burden and bacterial clearance in the spleens and livers of wild-type and CCR5−/− mice following primary infection with 2,000 L. monocytogenes organisms. On days 1 and 3 postinfection, no significant differences were observed between bacterial replication in the spleens and livers of CCR5-deficient and control animals. Control of L. monocytogenes replication requires successful activation of the early innate immune defenses, while complete bacterial clearance depends on the activation of adaptive immune responses. By day 15 postinfection, sterilizing immunity was achieved in the organs of both groups of mice (Fig. 1A), indicating that CCR5 was dispensable for both early and late stages of primary clearance of L. monocytogenes. We next examined the pattern of CCR5 expression in the infected organs of wild-type mice. On L. monocytogenes infection in the spleen, bacterium-containing lesions localize to the white pulp areas, where bacteria are associated with Mac-3+ cells. In the infected liver, bacterium-containing lesions are characterized by infiltration with F4/80+ macrophages (data not shown). Immunohistochemical analysis demonstrated significant infiltration of CCR5+ cells into the bacterium-containing lesions in the spleens and livers of wild-type mice. No CCR5 reactivity was observed in the tissues of CCR5-deficient mice (Fig. 1B and C).
FIG. 1.
L. monocytogenes infection in CCR5−/− mice. (A) C57BL/6 and CCR5−/− mice were infected intravenously with 2,000 live L. monocytogenes organisms, the spleens and livers were removed at the indicated times, and viable bacteria were quantified. Mean numbers of CFU from groups of three mice are shown. The experiment was repeated twice. (B and C) Organs were removed at 3 days postinfection, and frozen 5-μm sections were prepared. Spleen (B) and liver (C) sections were stained for CCR5 (red) and counterstained with hematoxylin.
Cell migration and activation of innate immunity in response to L. monocytogenes infection is normal in the absence of CCR5.
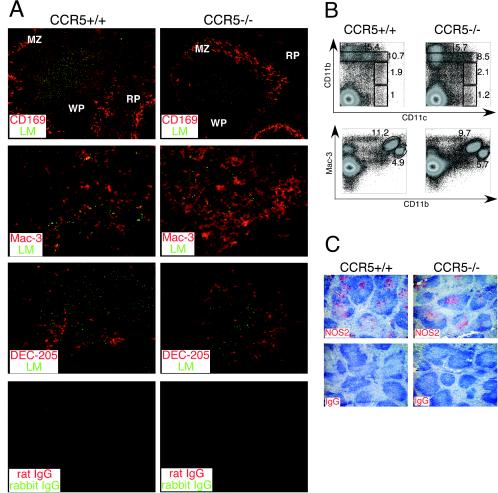
The spleen contains a rich supply of macrophages with distinct subpopulations that localize to the white pulp, red pulp, and marginal-zone areas. The functions of these various subsets of macrophages during the course of intracellular infection are not clear. Since CCR5 is expressed on macrophages and DCs, we examined the distribution of these cells in the spleen during L. monocytogenes infection of wild-type and CCR5-deficient mice. In both groups of mice, splenic marginal zones were characterized by the presence of CD169-positive macrophages and Listeria-containing lesions were localized to the white pulp areas (Fig. 2A). Bacteria in lesions were associated with cells expressing Mac-3, a marker expressed on subsets of macrophages and DCs, suggesting that CCR5 is dispensable for migration of these cells during infection (Fig. 2B). We next characterized various DC subsets in collagenase-digested spleens of infected wild-type and CCR5-deficient mice by flow cytometric analysis. Conventional mouse DCs are characterized by high expression of CD11c and low or negative expression of CD11b. Furthermore, CD11b-negative DCs express DEC-205 and CD8 antigens (38). Recently, we identified a TNF- and iNOS-producing subset of dendritic cells (Tip-DCs) that expresses intermediate levels of CD11b and CD11c, high levels of Mac-3, and DEC-205 or CD8 (35). Similar percentages of CD11chigh CD11blow and CD11chigh CD11bneg cells (conventional DCs) were present in the CCR5+/+ and CCR5−/− spleens at 48 h postinfection (Fig. 2B). Migration of Tip-DCs (CD11clow/int CD11bint Mac-3high) was also normal in CCR5-deficient spleens (Fig. 2B). During infection, DEC-205+ cells localize on the periphery of Listeria-containing lesions in the white pulp (35). Immunohistological analysis revealed that localization of DEC-205+ DCs in the white-pulp areas was also independent of CCR5 expression (Fig. 2A).
FIG.2.
Normal migration of macrophages and DCs in the absence of CCR5. (A) C57BL/6 and CCR5−/− mice were infected with 2,000 L. monocytogenes organisms, and spleens were harvested at 2 days postinfection. Spleen sections were stained with antibodies to L. monocytogenes (green), CD169 (red), Mac-3 (red), and DEC-205 (red). RP, red pulp; WP, white pulp; MZ, marginal zone. (B) Spleens were harvested from wild-type and CCR5−/− mice at 2 days postinfection and digested with collagenase, and the expression of CD11c, CD11b, and Mac-3 was analyzed by flow cytometry. The percentages of CD11bhigh, CD11bint, CD11blow CD11chigh, and CD11bneg CD11chigh (upper panels) and of CD11bint Mac-3high and CD11bhigh Mac-3low (lower panels) cells are indicated. Representative dot plots for three mice per group are shown. (C) Spleen sections from mice infected with L. monocytogenes for 3 days were stained for iNOS (red) and counterstained with hematoxylin. Each subpanel in panels A and C is a representative staining with three mice per group analyzed, and the experiments were repeated twice.
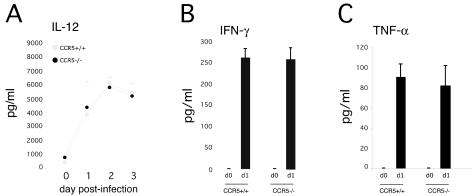
We next examined the activation of antimicrobial innate immune defenses in the absence of CCR5. On days 2 and 3 postinfection, iNOS expression was evident in the spleens of both groups of mice, indicating that CCR5 signaling was not required for the activation of Tip-DCs (Fig. 2C and data not shown). The specificity of anti-iNOS staining was confirmed by staining tissues from iNOS-deficient mice. No antibody reactivity was observed in the spleens of iNOS-deficient animals (data not shown). Robust IL-12 secretion in response to L. monocytogenes infection was observed in the spleens of CCR5-deficient mice (Fig. 3A). Likewise, expression of IFN-γ and TNF-α at 24 h postinfection was normal in spleens of CCR5−/− mice (Fig. 3B and C).
FIG. 3.
Cytokine production in the spleens of wild-type and CCR5−/− mice. C57BL/6 and CCR5−/− mice were infected with 2,000 L. monocytogenes organisms, and their spleens were removed at the indicated times and lysed in PBS containing 0.01% Triton X-100. IL-12 (A), IFN-γ (B), and TNF-α (C) in lysates were quantified by sandwich ELISA. Each time point represents three or four mice, and the experiment was repeated twice.
Adaptive immunity functions normally in the absence of CCR5.
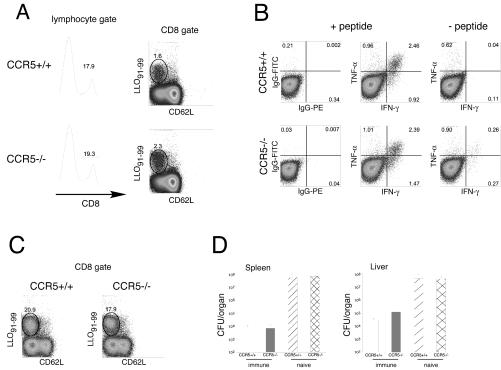
Memory CD8+ T cells express CCR5. To determine whether the absence of CCR5 affected the priming of L. monocytogenes-specific T cells and subsequent development of memory lymphocytes, we examined CD8+ T-cell responses to the immunodominant LLO91-99 epitope 9 days after primary infection. The percentages of total CD8+ T cells and LLO91-99-specific CD8+ cells were similar in the spleens of CCR5+/+ and CCR5−/− mice (Fig. 4A), suggesting that CCR5 was dispensable for the priming of Listeria-specific T cells. Intracellular cytokine staining revealed that LLO-specific, CD8+ T cells produced TNF-α and IFN-γ in response to peptide stimulation, indicating that differentiation of antigen-specific cells was also intact in the absence of CCR5 signaling (Fig. 4B). To examine the impact of CCR5 deficiency on recall responses, previously immunized wild-type and CCR5-deficient mice were rechallenged with L. monocytogenes 4 weeks after primary infection and the magnitude of LLO91-99-specific CD8-cell responses was measured 5 days later. Our results indicate that migration and expansion of memory CD8 T cells occurs normally in the absence of CCR5 (Fig. 4C). To confirm that CCR5-deficient memory CD8 T cells were fully functional, bacterial replication in the organs of rechallenged immune and acutely infected mice was examined. At 3 days after the rechallenge, bacterial titers in the spleens and livers of immune CCR5+/+ and CCR5−/− mice were significantly reduced (3 to 4 log units) as compared to those in acutely infected control mice (Fig. 4D), demonstrating intact immune memory in the absence of CCR5.
FIG. 4.
Normal primary and secondary CD8 T-cell responses in CCR5−/− mice. (A and B) C57BL/6-H2-Kd and CCR5−/−-H2-Kd mice were infected with 2,000 L. monocytogenes organisms, and their spleens were harvested at 9 days postinfection. (A) Splenocytes were stained for CD8, CD62L, and H2-Kd tetramer, gated on lymphocytes by size, further gated on CD8α, and analyzed by flow cytometry. (Left panels) CD8 staining in the lymphocyte gate. (Right panels) CD62L and H2-Kd tetramer staining of CD8α+ gated cells. The percentages of CD8α and tetramer-positive T cells are indicated. (B) Splenocytes from wild-type and CCR5−/− mice were incubated with or without LLO91-99 peptide in the presence of brefeldin A for 5 h, and intracellular TNF-α and IFN-γ staining was performed. The cells were gated on the CD62L−CD8α+ population. The percentage of cytokine-positive cells is indicated. (C) Immune C57BL/6-H2-Kd and CCR5−/−-H2-Kd mice were rechallenged with 100,000 L. monocytogenes organisms, and their spleens were harvested at 5 days postinfection. Splenocytes were stained as in panel A. For panels A to C, representative dot plots for three mice per group are shown. (D) Naive or immune C57BL/6-H2-Kd and CCR5−/−-H2-Kd mice were challenged with 100,000 L. monocytogenes organisms, their spleens and livers were harvested at 3 days postinfection, and the bacterial titers in the organs were determined. Each bar represents three or four mice.
CD8 T-cell migration to nonlymphoid organs in the absence of CCR5.
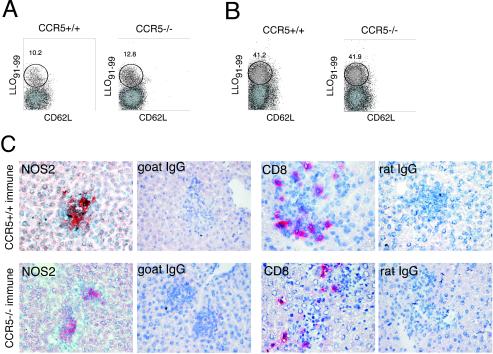
The spleen contains vast numbers of T lymphocytes. Activation of resident CD8 T cells in response to infection in the spleen might be normal, while migration of T cells to peripheral sites in the absence of CCR5 might be abnormal. Therefore, we examined T-cell immunity in the liver, a nonlymphoid organ that is readily infected with L. monocytogenes. Naive livers contain few T cells, and the percentages of CD4 and CD8 T cells increase significantly after infection (data not shown). By day 9 after the primary infection, all CD8 T cells in the livers of wild-type and CCR5 knockout mice were uniformly CD62L negative, with approximately 10 to 12% of T cells staining with the LLO91-99 tetramer (Fig. 5A). At 5 days after the secondary challenge, approximately 40% of CD8 T cells in the livers of immune wild-type and CCR5-deficient mice were LLO91-99 specific (Fig. 5B), suggesting that migration of memory T cells into nonlymphoid sites of inflammation does not require CCR5 expression.
FIG. 5.
CCR5 is dispensable for CD8 T-cell migration to peripheral sites. (A and B) Livers were harvested from naive C57BL/6-H2-Kd and CCR5−/−-H2-Kd mice infected with 2,000 L. monocytogenes organisms for 9 days (A) or immune mice infected with 100,000 L. monocytogenes organisms for 5 days (B), and splenocytes were stained for CD8, CD62L, and H2-Kd tetramer. The cells were gated on lymphocytes by size, further gated on CD8α, and analyzed by flow cytometry. (C) Livers were harvested from immune mice at 2 days after the rechallenge, and frozen sections were prepared. Sections were stained for iNOS and CD8a as indicated in the panels and counterstained with hematoxylin.
Next, livers of rechallenged immune wild-type and CCR5−/− mice were examined histologically. By day 2 after the rechallenge, livers from the CCR5+/+ and CCR5−/− mice contained numerous, well-organized bacterium-containing lesions that were characterized by the presence of activated, iNOS-positive macrophages. Lesions in both groups of mice were infiltrated with CD8 T cells (Fig. 5C), demonstrating that CCR5 is also not necessary for targeting of memory T cells to the sites of bacterial infection.
DISCUSSION
CCR5 is expressed on a variety of hematopoietic cell types including DCs, macrophages, and Th1-type T cells (23). However, it remains unclear whether CCR5 expression by these cell types has a biological significance during in vivo immune responses. Here, we provide a detailed characterization of innate and adaptive immune responses to L. monocytogenes in CCR5-deficient hosts. In our study, the putative roles of CCR5 in both cellular recruitment and activation under inflammatory conditions were examined. We demonstrate that recruitment of macrophages, DCs, and CD8 T cells during both primary and secondary immune responses to L. monocytogenes occurs normally in CCR5−/− mice. Furthermore, CCR5 is not required for the induction of TNF, IL-12, and IFN-γ during innate responses, activation of bactericidal mechanisms, and priming of Listeria-specific CD8 T cells.
Immediately following introduction of the infectious agent into the host environment, inflammatory events take place which are characterized by the establishment of chemotactic gradients redirecting normally circulating cells into the sites of injury. In vivo expression of MIP-1α in the spleens of L. monocytogenes-infected mice and in vitro expression of MIP-1α and MIP-1β by Listeria-infected bone marrow-derived macrophages has been reported (10, 15). In vitro, CCR5 ligation induces the migration of macrophages, immature DCs, and activated and memory Th1 T cells (23, 31). In vivo, injection of soluble T. gondii antigens induces CCR5-dependent migration of CD8α+ DEC-205+ DCs from the red pulp and marginal-zone areas of the spleen to T-cell-containing areas of the white pulp (1). During infection with Leishmania donovanii, the granulomatous response in the liver is impaired in the absence of CCR5, suggesting the presence of abnormalities in the cell trafficking (34). In contrast, we observed normal migration of DCs and macrophages and unimpaired formation of bacterium-containing lesions in spleens and livers during the anti-listerial immune response in CCR5-deficient mice.
It is intriguing that despite abundant CCR5 expression in the infected tissues, CCR5 deficiency had no apparent effect during the immune response to L. monocytogenes. One difficulty in understanding the roles of individual chemokines and chemokine receptors stems from their redundancy: many receptors have the same chemokine ligands, and many chemokines bind several chemokine receptors. Only one CCR5 ligand, MIP-1β, is unique to this receptor, while MIP-1α, RANTES, and MCP are shared with CCR1, CCR2, CCR3, and CCR4 (45). During L. monocytogenes infection, other receptors might be utilized by MIP-1α and RANTES in the absence of CCR5 in vivo, an issue that could be addressed only by generating mice with multiple chemokine receptor deficiencies.
In this respect, it is notable that mice lacking CCR2 have pronounced deficiencies in monocyte recruitment in vivo and are highly susceptible to L. monocytogenes infection. CCR2 is the sole receptor for its major ligand, MCP-1 (45). Thus, redundancy in the use of chemokine receptors might be a correlate of how well the particular chemokine deficiency will be tolerated during the inflammation. Additionally, the relative importance of individual chemokine receptors during inflammatory reactions might depend strongly on the preferential site for parasite multiplication. Successful immunity to C. neoformans requires CCR5 expression (19). However, the susceptibility is associated with the defective recruitment of leukocytes to the brains of C. neoformans-infected mice while cellular migration to the lungs appears intact, suggesting the existence of tissue-specific requirements for CCR5.
In addition to inducing cellular migration, other biological functions have been ascribed to CCR5 and its ligands. In vitro, MIP-1α induces the secretion of TNF, IL-6, and IL-1 by peritoneal macrophages, suggesting a role for this chemokine in the direct activation of cells (14). In line with these findings, CCR5-deficient peritoneal macrophages were reported to have partial defects in the production of IL-6 and IL-1 in response to MIP-1α (44). In vivo, administration of a tachyzoite antigen extraxt (STAg) to mice induced strong CCR5-driven IL-12 production by CD8α+ DCs (1). Recently, it was shown that TNF and IL-12 secretion by L. monocytogenes-infected bone marrow-derived macrophages in response to IFN-γ in vitro is enhanced by treatment with MIP-1α, MIP-1β, and RANTES, suggesting synergism between Th1-type cytokines and CC chemokines (11). However, the latter in vitro findings do not appear to correlate with our in vivo observations, since there was no reduction in the levels of TNF, IL-12, or IFN-γ during primary infection of CCR5-deficient mice with L. monocytogenes. In agreement with normal cytokine secretion, expression of iNOS was also intact, indicating that the induction of bactericidal functions was unimpaired. Interestingly, T. gondii cyclophilin has been shown to bind CCR5, mimicking its chemokine ligands and triggering cytokine secretion and DC migration, suggesting that some pathogens might actively exploit the expression of chemokine receptors on inflammatory cells (2).
In addition to its expression on cells of the innate immune system, CCR5 expression on effector and memory T cells has been reported (16, 25, 30, 31). In vitro, MIP-1α, MIP-1β, and RANTES enhance antigen-specific T-cell proliferation and IL-2 production (41). All CCR5 ligands were demonstrated to be chemotactic for Th1-type T cells, and MIP-1α was shown to induce the migration of activated CD8 T cells (40). Lymphocytes migrating to the liver in response to hepatitis C virus infection express CCR5, and it was suggested that tissue infiltration by lymphocytes correlates with the high level of CCR5 expression (36). However, most of our understanding of the role of CCR5 expression on T cells comes from in vitro chemotaxis experiments and the phenotypic analysis of peripheral lymphocytes. Our study directly addressed the role of CCR5 in memory T-cell migration to the sites of inflammation during in vivo bacterial infection. Recall CD8 T-cell responses in the spleens and livers of L. monocytogenes-infected CCR5−/− mice were comparable to those observed in wild-type control mice, suggesting that CCR5 expression by memory T cells is not essential for their migration. Other chemokine receptors such as CCR2 and CXCR3 may recruit memory T cells to the sites of inflammation in the absence of CCR5. Additionally, the presence of long-lived memory cells in the nonlymphoid tissues has been shown (24), suggesting that the need for chemokine-mediated cellular recruitment might be bypassed in these tissues by directly activating resident cells.
CCR5 is a major coreceptor used by M-tropic strain of HIV-1 (3, 9, 12). Individuals homozygous for the mutant allele, CCR5-Δ32, are highly resistant to HIV infection, and heterozygotes show delayed disease progression (34). CCR5 ligands can prevent the entry of HIV-1 into cells, suggesting that strategies limiting the normal availability of surface CCR5 to viral gp120 are highly promising in reducing HIV infectivity (7). However, the expression of CCR5 on cells critical for both innate and adaptive immune responses raises the question of the impact of CCR5 blockade on the immune responses to other pathogens. To date, the in vivo role of CCR5 has been characterized only with respect to fungal (C. neoformans) and protozoan (T. gondii) pathogens (1, 2, 19). Our study provides the first comprehensive examination of the in vivo immune responses to an intracellular bacterial pathogen in the absence of CCR5 and suggests that CCR5 is dispensable for both migration and activation of cells during primary and secondary immune responses to L. monocytogenes. Although the susceptibility of CCR5-deficient mice to other bacterial pathogens remains to be tested, our data correlate with the finding that CCR5-deficient humans are not detectably more susceptible to bacterial infections.
Acknowledgments
This work was supported by National Institutes of Health grants RO1 AI 42135 and AI 39031 (E.G.P.) and National Institutes of Health research training grant CA 09149 (N.V.S.)
Editor: F. C. Fang
REFERENCES
- 1.Aliberti, J., C. Reis e Sousa, M. Schito, S. Hieny, T. Wells, G. B. Huffnagle, and A. Sher. 2000. CCR5 provides a signal for microbial induced production of IL-12 by CD8 alpha+ dendritic cells. Nat. Immunol. 1:83-87. [DOI] [PubMed] [Google Scholar]
- 2.Aliberti, J., J. G. Valenzuela, V. B. Carruthers, S. Hieny, J. Andersen, H. Charest, C. Reis e Sousa, A. Fairlamb, J. M. Ribeiro, and A. Sher. 2003. Molecular mimicry of a CCR5 binding-domain in the microbial activation of dendritic cells. Nat. Immunol. 4:485-490. [DOI] [PubMed] [Google Scholar]
- 3.Alkhatib, G., C. Combadiere, C. C. Broder, Y. Feng, P. E. Kennedy, P. M. Murphy, and E. A. Berger. 1996. CC CKR5: a RANTES, MIP-1alpha, MIP-1beta receptor as a fusion cofactor for macrophage-tropic HIV-1. Science 272:1955-1958. [DOI] [PubMed] [Google Scholar]
- 4.Bielecki, J., P. Youngman, P. Connelly, and D. A. Portnoy. 1990. Bacillus subtilis expressing a haemolysin gene from Listeria monocytogenes can grow in mammalian cells. Nature 345:175-176. [DOI] [PubMed] [Google Scholar]
- 5.Buchmeier, N. A., and R. D. Schreiber. 1985. Requirement of endogenous interferon-gamma production for resolution of Listeria monocytogenes infection. Proc. Natl. Acad. Sci. USA 82:7404-7408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Busch, D. H., I. M. Pilip, S. Vijh, and E. G. Pamer. 1998. Coordinate regulation of complex T cell populations responding to bacterial infection. Immunity 8:353-362. [DOI] [PubMed] [Google Scholar]
- 7.Cocchi, F., A. L. DeVico, A. Garzino-Demo, S. K. Arya, R. C. Gallo, and P. Lusso. 1995. Identification of RANTES, MIP-1 alpha, and MIP-1 beta as the major HIV-suppressive factors produced by CD8+ T cells. Science 270:1811-1815. [DOI] [PubMed] [Google Scholar]
- 8.Combadiere, C., S. K. Ahuja, H. L. Tiffany, and P. M. Murphy. 1996. Cloning and functional expression of CC CKR5, a human monocyte CC chemokine receptor selective for MIP-1(alpha), MIP-1(beta), and RANTES. J. Leukoc. Biol. 60:147-152. [DOI] [PubMed] [Google Scholar]
- 9.Deng, H., R. Liu, W. Ellmeier, S. Choe, D. Unutmaz, M. Burkhart, P. Di Marzio, S. Marmon, R. E. Sutton, C. M. Hill, C. B. Davis, S. C. Peiper, T. J. Schall, D. R. Littman, and N. R. Landau. 1996. Identification of a major co-receptor for primary isolates of HIV-1. Nature 381:661-666. [DOI] [PubMed] [Google Scholar]
- 10.DiTirro, J., E. R. Rhoades, A. D. Roberts, J. M. Burke, A. Mukasa, A. M. Cooper, A. A. Frank, W. K. Born, and I. M. Orme. 1998. Disruption of the cellular inflammatory response to Listeria monocytogenes infection in mice with disruptions in targeted genes. Infect. Immun. 66:2284-2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dorner, B. G., A. Scheffold, M. S. Rolph, M. B. Huser, S. H. Kaufmann, A. Radbruch, I. E. Flesch, and R. A. Kroczek. 2002. MIP-1alpha, MIP-1beta, RANTES, and ATAC/lymphotactin function together with IFN-gamma as type 1 cytokines. Proc. Natl. Acad. Sci. USA 99:6181-6186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dragic, T., V. Litwin, G. P. Allaway, S. R. Martin, Y. Huang, K. A. Nagashima, C. Cayanan, P. J. Maddon, R. A. Koup, J. P. Moore, and W. A. Paxton. 1996. HIV-1 entry into CD4+ cells is mediated by the chemokine receptor CC-CKR-5. Nature 381:667-673. [DOI] [PubMed] [Google Scholar]
- 13.Edelson, B. T., and E. R. Unanue. 2000. Immunity to Listeria infection. Curr Opin Immunol 12:425-31. [DOI] [PubMed] [Google Scholar]
- 14.Fahey, T. J., III, K. J. Tracey, P. Tekamp-Olson, L. S. Cousens, W. G. Jones, G. T. Shires, A. Cerami, and B. Sherry. 1992. Macrophage inflammatory protein 1 modulates macrophage function. J. Immunol. 148:2764-2769. [PubMed] [Google Scholar]
- 15.Flesch, I. E., J. Barsig, and S. H. Kaufmann. 1998. Differential chemokine response of murine macrophages stimulated with cytokines and infected with Listeria monocytogenes. Int. Immunol. 10:757-765. [DOI] [PubMed] [Google Scholar]
- 16.Fukada, K., Y. Sobao, H. Tomiyama, S. Oka, and M. Takiguchi. 2002. Functional expression of the chemokine receptor CCR5 on virus epitope-specific memory and effector CD8+ T cells. J. Immunol. 168:2225-2232. [DOI] [PubMed] [Google Scholar]
- 17.Gong, W., O. M. Howard, J. A. Turpin, M. C. Grimm, H. Ueda, P. W. Gray, C. J. Raport, J. J. Oppenheim, and J. M. Wang. 1998. Monocyte chemotactic protein-2 activates CCR5 and blocks CD4/CCR5-mediated HIV-1 entry/replication. J. Biol. Chem. 273:4289-4292. [DOI] [PubMed] [Google Scholar]
- 18.Harty, J. T., and M. J. Bevan. 1995. Specific immunity to Listeria monocytogenes in the absence of IFN gamma. Immunity 3:109-117. [DOI] [PubMed] [Google Scholar]
- 19.Huffnagle, G. B., L. K. McNeil, R. A. McDonald, J. W. Murphy, G. B. Toews, N. Maeda, and W. A. Kuziel. 1999. Cutting edge: role of C-C chemokine receptor 5 in organ-specific and innate immunity to Cryptococcus neoformans. J. Immunol. 163:4642-4646. [PubMed] [Google Scholar]
- 20.Kerksiek, K. M., A. Ploss, I. Leiner, D. H. Busch, and E. G. Pamer. 2003. H2-M3-restricted memory T cells: persistence and activation without expansion. J. Immunol. 170:1862-1869. [DOI] [PubMed] [Google Scholar]
- 21.Kuziel, W. A., T. C. Dawson, M. Quinones, E. Garavito, G. Chenaux, S. S. Ahuja, R. L. Reddick, and N. Maeda. 2003. CCR5 deficiency is not protective in the early stages of atherogenesis in apoE knockout mice. Atherosclerosis 167:25-32. [DOI] [PubMed] [Google Scholar]
- 22.Liu, R., W. A. Paxton, S. Choe, D. Ceradini, S. R. Martin, R. Horuk, M. E. MacDonald, H. Stuhlmann, R. A. Koup, and N. R. Landau. 1996. Homozygous defect in HIV-1 coreceptor accounts for resistance of some multiply-exposed individuals to HIV-1 infection. Cell 86:367-377. [DOI] [PubMed] [Google Scholar]
- 23.Luther, S. A., and J. G. Cyster. 2001. Chemokines as regulators of T cell differentiation. Nat. Immunol. 2:102-107. [DOI] [PubMed] [Google Scholar]
- 24.Masopust, D., V. Vezys, A. L. Marzo, and L. Lefrancois. 2001. Preferential localization of effector memory cells in nonlymphoid tissue. Science 291:2413-2417. [DOI] [PubMed] [Google Scholar]
- 25.Nansen, A., O. Marker, C. Bartholdy, and A. R. Thomsen. 2000. CCR2+ and CCR5+ CD8+ T cells increase during viral infection and migrate to sites of infection. Eur. J. Immunol. 30:1797-1806. [DOI] [PubMed] [Google Scholar]
- 26.Oxenius, A., U. Karrer, R. M. Zinkernagel, and H. Hengartner. 1999. IL-12 is not required for induction of type 1 cytokine responses in viral infections. J. Immunol. 162:965-973. [PubMed] [Google Scholar]
- 27.Pasparakis, M., L. Alexopoulou, V. Episkopou, and G. Kollias. 1996. Immune and inflammatory responses in TNF alpha-deficient mice: a critical requirement for TNF alpha in the formation of primary B cell follicles, follicular dendritic cell networks and germinal centers, and in the maturation of the humoral immune response. J. Exp. Med. 184:1397-1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Raport, C. J., J. Gosling, V. L. Schweickart, P. W. Gray, and I. F. Charo. 1996. Molecular cloning and functional characterization of a novel human CC chemokine receptor (CCR5) for RANTES, MIP-1beta, and MIP-1alpha. J. Biol. Chem. 271:17161-17166. [DOI] [PubMed] [Google Scholar]
- 29.Ruffing, N., N. Sullivan, L. Sharmeen, J. Sodroski, and L. Wu. 1998. CCR5 has an expanded ligand-binding repertoire and is the primary receptor used by MCP-2 on activated T cells. Cell. Immunol. 189:160-168. [DOI] [PubMed] [Google Scholar]
- 30.Sallusto, F., D. Lenig, C. R. Mackay, and A. Lanzavecchia. 1998. Flexible programs of chemokine receptor expression on human polarized T helper 1 and 2 lymphocytes. J. Exp. Med. 187:875-883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sallusto, F., C. R. Mackay, and A. Lanzavecchia. 2000. The role of chemokine receptors in primary, effector, and memory immune responses. Annu. Rev. Immunol. 18:593-620. [DOI] [PubMed] [Google Scholar]
- 32.Samson, M., O. Labbe, C. Mollereau, G. Vassart, and M. Parmentier. 1996. Molecular cloning and functional expression of a new human CC-chemokine receptor gene. Biochemistry 35:3362-3367. [DOI] [PubMed] [Google Scholar]
- 33.Samson, M., F. Libert, B. J. Doranz, J. Rucker, C. Liesnard, C. M. Farber, S. Saragosti, C. Lapoumeroulie, J. Cognaux, C. Forceille, G. Muyldermans, C. Verhofstede, G. Burtonboy, M. Georges, T. Imai, S. Rana, Y. Yi, R. J. Smyth, R. G. Collman, R. W. Doms, G. Vassart, and M. Parmentier. 1996. Resistance to HIV-1 infection in caucasian individuals bearing mutant alleles of the CCR-5 chemokine receptor gene. Nature 382:722-725. [DOI] [PubMed] [Google Scholar]
- 34.Sato, N., W. A. Kuziel, P. C. Melby, R. L. Reddick, V. Kostecki, W. Zhao, N. Maeda, S. K. Ahuja, and S. S. Ahuja. 1999. Defects in the generation of IFN-gamma are overcome to control infection with Leishmania donovani in CC chemokine receptor (CCR) 5-, macrophage inflammatory protein-1 alpha-, or CCR2-deficient mice. J. Immunol. 163:5519-5525. [PubMed] [Google Scholar]
- 35.Serbina, N., T. P. Salazar-Mather, C. Biron, W. A. Kuziel, and E. G. Pamer. 2003. TNF/iNOS-producing dendritic cells mediate innate immune defense against bacterial infection. Immunity 19:59-70. [DOI] [PubMed] [Google Scholar]
- 36.Shields, P. L., C. M. Morland, M. Salmon, S. Qin, S. G. Hubscher, and D. H. Adams. 1999. Chemokine and chemokine receptor interactions provide a mechanism for selective T cell recruitment to specific liver compartments within hepatitis C-infected liver. J. Immunol. 163:6236-6243. [PubMed] [Google Scholar]
- 37.Shiloh, M. U., J. D. MacMicking, S. Nicholson, J. E. Brause, S. Potter, M. Marino, F. Fang, M. Dinauer, and C. Nathan. 1999. Phenotype of mice and macrophages deficient in both phagocyte oxidase and inducible nitric oxide synthase. Immunity 10:29-38. [DOI] [PubMed] [Google Scholar]
- 38.Shortman, K., and Y. J. Liu. 2002. Mouse and human dendritic cell subtypes. Nat. Rev. Immunol. 2:151-161. [DOI] [PubMed] [Google Scholar]
- 39.Simmons, G., J. D. Reeves, S. Hibbitts, J. T. Stine, P. W. Gray, A. E. Proudfoot, and P. R. Clapham. 2000. Co-receptor use by HIV and inhibition of HIV infection by chemokine receptor ligands. Immunol. Rev. 177:112-126. [DOI] [PubMed] [Google Scholar]
- 40.Taub, D. D., K. Conlon, A. R. Lloyd, J. J. Oppenheim, and D. J. Kelvin. 1993. Preferential migration of activated CD4+ and CD8+ T cells in response to MIP-1 alpha and MIP-1 beta. Science 260:355-358. [DOI] [PubMed] [Google Scholar]
- 41.Taub, D. D., S. M. Turcovski-Corrales, M. L. Key, D. L. Longo, and W. J. Murphy. 1996. Chemokines and T lymphocyte activation. I. Beta chemokines costimulate human T lymphocyte activation in vitro. J. Immunol. 156:2095-2103. [PubMed] [Google Scholar]
- 42.Tripp, C. S., M. K. Gately, J. Hakimi, P. Ling, and E. R. Unanue. 1994. Neutralization of IL-12 decreases resistance to Listeria in SCID and C.B-17 mice. Reversal by IFN-gamma. J. Immunol. 152:1883-1887. [PubMed] [Google Scholar]
- 43.Wong, P., and E. G. Pamer. 2003. CD8 T cell responses to infectious pathogens. Annu. Rev. Immunol. 21:29-70. [DOI] [PubMed] [Google Scholar]
- 44.Zhou, Y., T. Kurihara, R. P. Ryseck, Y. Yang, C. Ryan, J. Loy, G. Warr, and R. Bravo. 1998. Impaired macrophage function and enhanced T cell-dependent immune response in mice lacking CCR5, the mouse homologue of the major HIV-1 coreceptor. J. Immunol. 160:4018-4025. [PubMed] [Google Scholar]
- 45.Zlotnik, A., and O. Yoshie. 2000. Chemokines: a new classification system and their role in immunity. Immunity 12:121-127. [DOI] [PubMed] [Google Scholar]