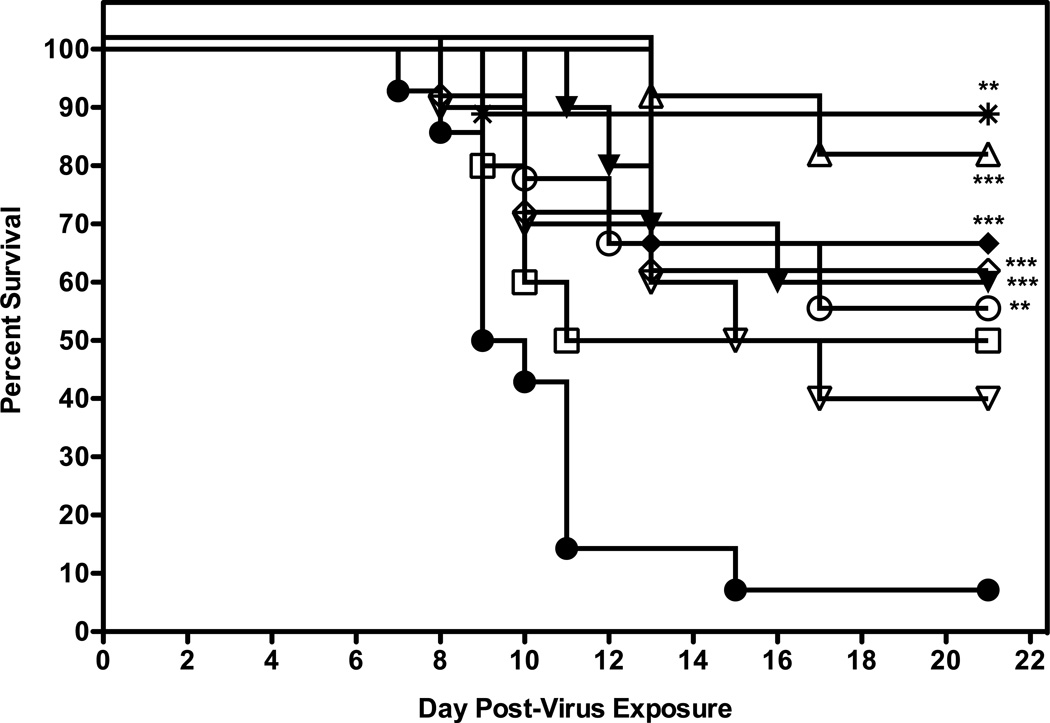
Figure 5.
Evaluation of different dosing regimens of 2’FdC in 0.4% CMC for efficacy against a highly pathogenic influenza H5N1 virus infection in BALB/c mice. Vehicle placebo, 2’-deoxy-2’-fluorocytidine, or ribavirin were administered intraperitoneally. Experimental groups, n = 10; placebo group, n = 15. 0.4% CMC (●), 60 (▲) and 30 (♦) mg/kg/day three times a day (tid); 60 (○) and 30 (□) mg/kg/day, once a day (qd) 24 h prior to challenge with virus; 60 mg/kg/day twice a day 24 h post virus exposure (Δ), 48 h post virus exposure (▽), 72 h (◇) post virus exposure, or ribavirin at 75 mg/kg/day (✴). All were administered a total of 8 days, regardless of time of addition.
*** P=0.0005, 2'-FdC (60 mg/kg, tid, −24 h); *** P=0.0005, 2'-FdC (30 mg/kg, tid, −24 h); ** P=0.0048, 2'-FdC (60 mg/kg, qd, −24 h); *** P= 0.0001, 2'-FdC (60 mg/kg, bid, +24 h); ** P= 0.0019, 2'-FdC (60 mg/kg, bid, +72 h); **P=0.0013, ribavirin (75 mg/kg, bid,−24 h) all versus 0.4% CMC.