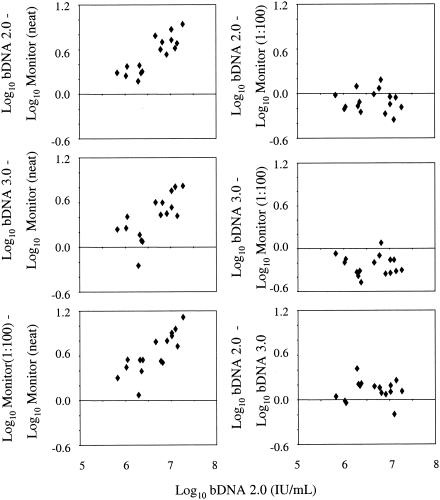
FIG. 1.
Evaluation of high-titer clinical specimens with Bayer bDNA 2.0, Bayer bDNA 3.0, Roche COBAS Monitor v. 2.0 undiluted (neat), and Roche COBAS Monitor v. 2.0 diluted 1:100. Log10-transformed bDNA 2.0 HCV RNA values are shown on the x axis. Differences between the HCV RNA values obtained by the bDNA 2.0, bDNA 3.0, and COBAS Monitor 1:100 assays compared to values obtained with the COBAS Monitor undiluted assay are shown in the left panels. Differences in HCV RNA levels obtained by the bDNA version 2.0 and 3.0 and COBAS Monitor 1:100 assays are shown in the right panels.