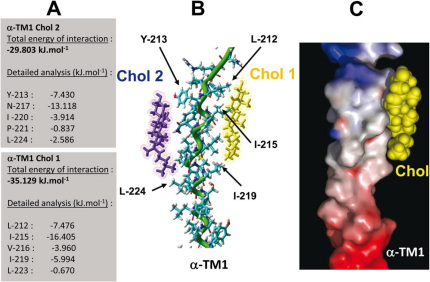
Figure 2. Docking of two cholesterol molecules on human AChR α-TM1.
Two cholesterol molecules (Chol 1 and Chol 2) were docked on the αTM1 domain of the human AChR as described in Materials and Methods. A detailed analysis of the energy of interaction of each cholesterol-TM1 complex is shown on the left panel (A). A tube rendering of the model is shown in (B). The α-helix of the TM domain is colored in green. The apolar part of Chol 1 (in yellow) interacts with Ile212, Ile215, and Ile219 (van der Waals interactions), whereas its polar part (the OH group) is located at the apolar-polar interface of the membrane where it can interact with water molecules. The OH group of Chol 2 (in violet) forms an H-bond with the phenolic OH of Tyr213. The surface complementarity between the α-TM1 domain and Chol 2 (in yellow) is illustrated by a surface view of the complex (C). The electrostatic surface of the α-TM1 domain is rendered in blue for positive, red for negative, and grey for neutral regions, respectively.