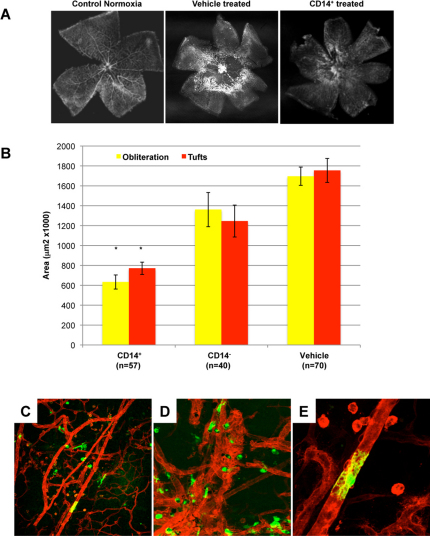
Figure 1. CD14+ cells stabilize and promote normalization of ischemia-injured retinal vasculature in the OIR model.
A) CD14+ cells normalize angiogenesis during hyperoxia and accelerate retinal revascularization. Normal retinas were dissected from C57BL/6J mice and stained with GS lectin at postnatal day 17 (P17). They show a characteristic branching vascular pattern radiating outward from the central optic nerve head (“Control Normoxia”). Exposure to hyperoxia for 5 days (from P7 to P12) leads to central vaso-obliteration. Once the mice return to normoxia, neovascularisation occurs at the interface between perfused peripheral, and non-perfused central, retina. Treatment with vehicle (“Vehicle treated”) at P7 does not alter the vaso-obliteration or neovascularisation. In contrast, treatment at P7 with hUCB-derived CD14+ cells leads to normalization of the retinal vasculature (“CD14+ treated”). B) Quantification of retinas treated with hUCB-derived cell populations. Retinas were analyzed at P17 using GS-lectin staining for retinal vessel obliteration (yellow bars) and tuft formation (neovascularization) (red bars) in retinal whole mounts. No significant difference was observed between vehicle and CD14− cells when injected intravitreally at P7. Obliteration and neovascularization are reduced by 63% and 56% respectively, compared to vehicle-treated retinas (n = 57, n = 40, n = 70, n = number of eyes) (*P <0.001 Bonferroni corrected t-test). C) D) And E) Ad5F16-eGFP hUCB-derived CD14+ cells (green) target and differentiate along the mouse retina vasculature (red) at P17 (10x, 20x and 40X respectively).