Figure 6. GFP-Sec16B interacts with Sec16A and Sec13.
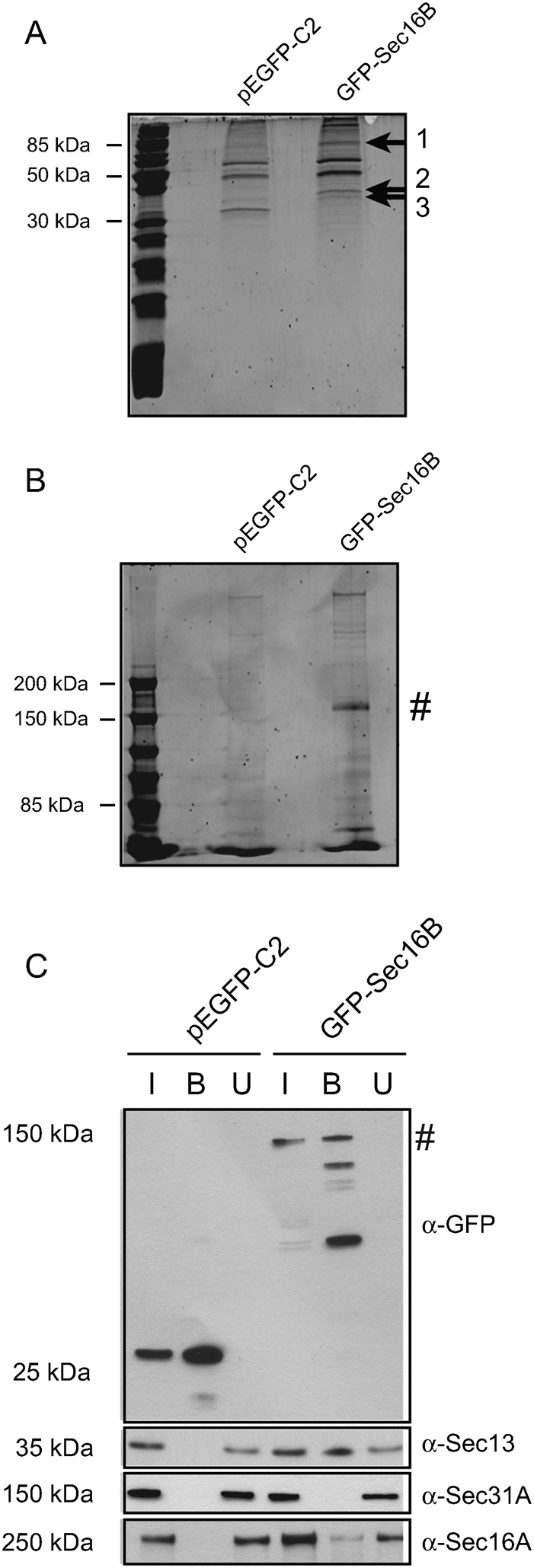
GFP-Sec16B and proteins bound to Sec16B were extracted from whole cell lysate using GFP-Trap®. The beads fraction was loaded on a 15% (A) and 5% (B) gel and subjected to SDS-PAGE to separate the proteins bound to GFP-Sec16B. The proteins were stained using SYPRO® Ruby. Three distinct protein bands were extracted from the 15% gel (1, 2 and 3, arrows), the proteins digested and analyzed by MS/MS spectrometry. The proteins were then identified by blast search against a human database. GFP-Sec16B migrated at ∼ 144 kD (B, #). (C) Identification of interaction partners of GFP-Sec16B by immunoprecipitation and immunoblotting. HeLa GFP-Sec16B cells and HeLa control cells expressing GFP were lysed. GFP and GFP-Sec16B were subsequently bound to GFP-Trap® beads. 1% of input and unbound fraction and 20% of beads were loaded. Probing with an anti-GFP antibody revealed some degradation of GFP-Sec16B during the sample analysis. Western blotting revealed that GFP-Sec16B (∼ 144 kD, #) and the GFP control were bound efficiently to the GFP-Trap® beads (top row, lane 2 and 5). GFP-Sec16B bound to Sec13 (second row, lane 5) and Sec16A (bottom row, lane 5), but not to Sec31A (third row, lane 5).
