Figure 1. Comparison of crystal structures of Bombyx mori JHBP in the apo- and JH-bound forms reveals a gate-latch mechanism for JH binding to JHBP.
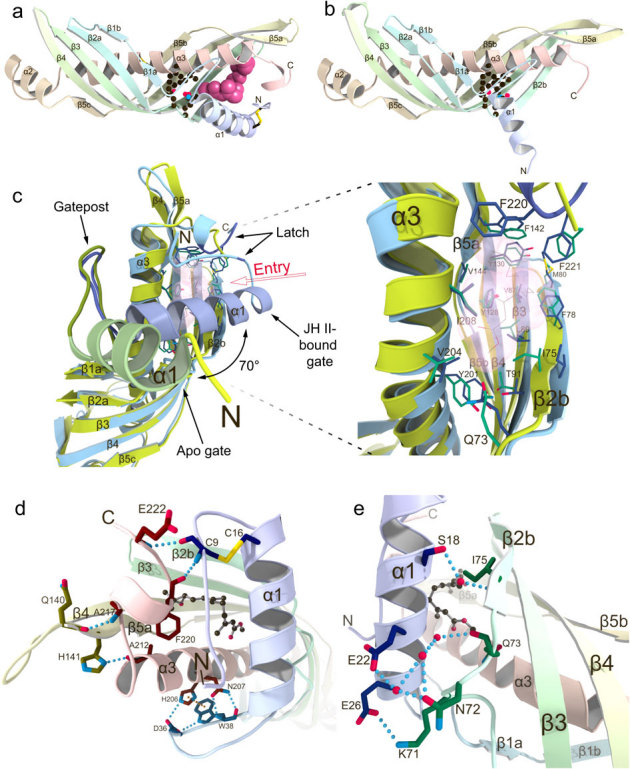
(a) The crystal structure of JHBP in complex with JH II. The bound JH II molecule is shown as a space-filling model. Side chains of residues forming the bottom wall of the JH-binding pocket are shown as ball-and-stick models and disulfide bonds as stick models. (b) The crystal structure of apo-JHBP. Side chains of residues forming the bottom wall of the JH-binding pocket are shown as ball-and-stick models and disulfide bonds as stick models. (c) Close-up views of the JH entry site in the overlay of the apo- (green) and JH II-bound (light blue) JHBP structures with the transparent surface of the JH II, showing movement of the gate α1 helix upon JH-binding as well as the conserved positioning of the JH-interacting residues in the protein core of the both forms. (d) Interactions between the latch-forming N-terminal arm and C-terminal tail that are further linked to the gate α1 helix and the protein core, respectively. Also shown is association of the α1-β1 connecting appendix-like loop with α3-helix. Key residues for interactions are shown as stick models with hydrogen bonds (light-blue dotted lines) and π-π stacking (orange dotted line). (e) Association of the gate α1 helix with β2 strand in the JHBP-JH II complex. The side chains of key residues for interactions are shown as stick models with hydrogen bonds (light-blue dotted lines). Water molecules are shown as red spheres and JH II as a ball-and-stick model.
