Figure 5. Proposed mechanism for JHBP-mediated JH transport in the hemolymph from corpora allata cells to target cells.
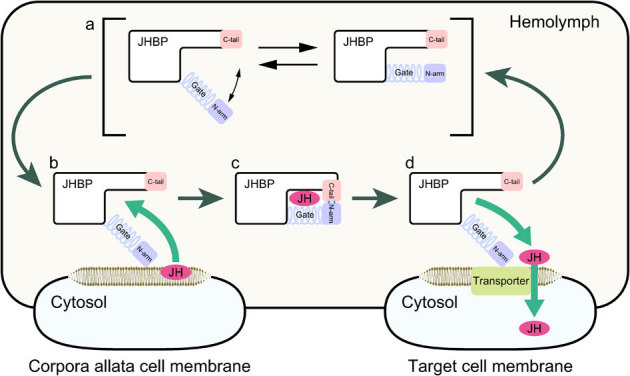
(a) In the ligand-free form, the JH carrier protein JHBP assumes various conformations of the gate α1 helix ranging from close to open forms. The open form presents a wide open, accessible cavity for JH with the flexible gate α1 and latch-like C-tail that guard the cavity's entrance. (b) JH binding at the membrance surface of the corpora allata cells where the JH is synthesized initiates an allosteric open-to-close transition, allowing the C-tail to form hydrogen bonds with the N-arm. (c) In the resulting fully closed JHBP-JH complex, the bound JH is completely buried inside the protein and protected from unfavorable nonspecific absorption and enzymatic degradation during the delivery to target cells in the hemolymph. (d) When the complex reaches to target cells, JHBP undergoes a conformational change from the fully closed to open forms that may be triggered by reduction of dielectric constant, and releases the bound JH.
