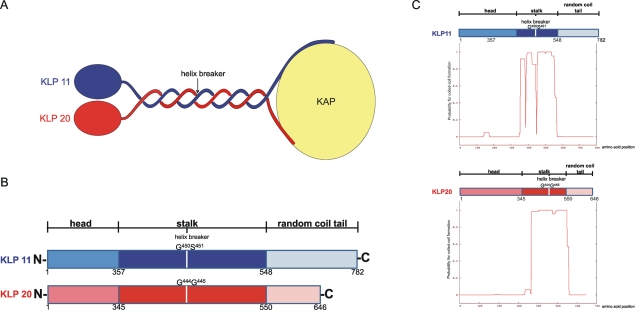
FIGURE 1:
Kinesin-2 architecture. (A) Schematic overview of the C. elegans heterotrimeric kinesin-2. KLP11 and KLP20 form a heterodimer that C-terminally associates with the cargo binding subunit, Kinesin Associated Protein (KAP). (B) Linear maps of the two motor subunits. The head, coiled-coil stalk, and RC domains are shown together with the amino acid positions that delimit their borders. The helix breaker positions are indicated in both motor domains; they allow folding of the tail onto the head domains to autoregulate catalytic activity. (C) Coiled-coil predictions for KLP11 and KLP20. Virtually the entire stalk region in KLP11 and KLP20 is predicted to form a coiled-coil (Lupas et al., 1991). The predictions are, however, limited to homodimeric coiled-coil formation.