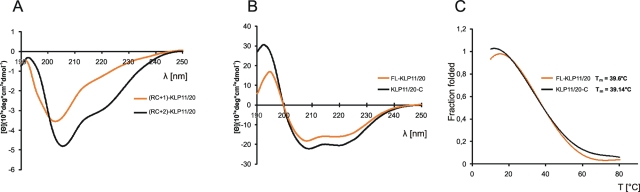
FIGURE 6:
CD spectroscopy indicates an increasing coiled-coil fraction with growing chain length under native conditions. (A) Spectra of (RC+1)-KLP11/20 and (RC+2)-KLP11/20 are dominated by an RC morphology (minimum at 195 nm) due to a low α-helical content. Note the increase in coiled-coil stability from RC+1 to RC+2 achieved with only eight more amino acids. Not only does the first minimum shift toward 208 nm, but also the minimum at 222 nm becomes more prominent, both signatures indicative of increasing secondary structure. (B) KLP11/20-C and FL-KLP11/20 show typical spectra of α-helical coiled-coil with double minima at 208 and 222 nm and a maximum at 195 nm. (C) Melting curves were recorded by following the change of ellipticity at 222 nm between 10°C and 80°C. The fraction of folded protein was calculated as ff = ([θ] – [θ]u)/([θ]n – [θ]u), where [θ]n and [θ]u represent the ellipticity values for the fully folded and fully unfolded species, respectively, and [θ] the observed ellipticity at 222 nm at any temperature. The comparable melting temperatures for KLP11/20-C and FL-KLP11/20 indicate low stability of the coiled-coil in the N-terminal half of the stalk.