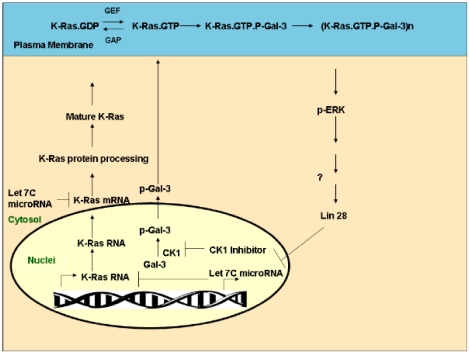
Figure 6. Gal-3 mediates cross-talk between K-Ras and Let 7c mirRNA: Proposed model.
Nuclear Gal-3 is phosphorylated by CK1 (p-Gal-3) and transported to the cytosol [20]. p-Gal-3 is then recruited to the plasma membrane (PM) where it interacts with K-Ras.GTP [10], [11], [18], which was formed by a guanine nucleotide exchange factor (GEF). K-Ras.GTP interacts with p-Gal-3, forming a complex that drives the formation of K-Ras nanoclusters [18]. These nanoclusters send strong Ras signals (e.g. to ERK). The signals might positively regulate the protein Lin 28, which binds and inhibits let-7c formation [39]. Because let-7c inhibits K-Ras translation, the Ras signal leads to relief of this inhibition and thus to enhanced translation of K-Ras. The translated K-Ras protein is processed in the cytosol to a mature protein, which is transported to the plasma membrane [40]–[42]. Once bound to GTP, it interacts with p-Gal-3 to form nanoclusters [18]. Accordingly, when p-Gal-3 is removed, either by knockdown of Gal-3 or by inhibition of CK1 [21], [33], Gal-3 cannot exert its positive control over K-Ras. This model is in accordance with the biological data on tumors that express high levels of Gal-3 [10], [11], [19] or low levels of let 7c [4]–[6] and which are highly aggressive.