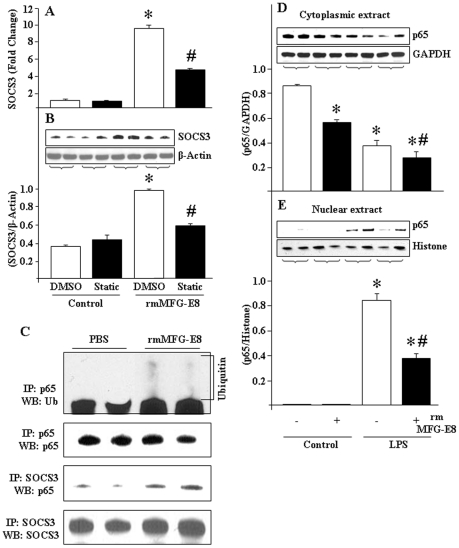
Figure 6. Interaction between NF-κB p65 and SOCS3 in rmMFG-E8-treated RAW264.7 cells.
(A, B) RAW264.7 cells (4×106 cells) were grown into the 6-well culture plates and incubated for overnight in presence of Static (6.25 µM), followed by rmMFG-E8 (500 ng/mL) treatment for 2 h. Real-time PCR was done for SOCS3 mRNA expression (A) and Western blotting for SOCS3 protein levels (B). In each samples, the expression of β-actin served as an internal control. (B) The protein level of SOCS3 was assessed by Western-blot from equal amounts (25 µg) of proteins and the image shown is representative of 3 independent experiments. The immunoblot was reprobed with mouse anti-β-actin Ab as loading control. Densitometric evaluations (SOCS3/β-actin) are expressed as means ± SE and compared by one-way ANOVA and SNK method: *P<0.05 vs. DMSO Control; # P<0.05 vs. DMSO+rmMFG-E8. (C) RAW264.7 cells (4×106 cells) were cultured in a 6-well cell culture plates, and treated with rmMFG-E8 (500 ng/mL) for 2 h. Proteins (80–100 µg) were immunoprecipitated using anti-mouse SOCS3 Ab and anti-mouse p65 Ab then subjected to Western-blotting and immunoreacted with anti-p65 Ab for the detection of p65 protein and anti-ubiquitin Ab for detection of ubiquitination. The membrane was re-probed with mouse anti-SOCS3 and anti-p65 Ab for determining SOCS3 and p65 contents. (D, E) RAW264.7 cells (4×106 cells) were grown in 6-well culture plates, and then pre-treated with rmMFG-E8 (500 ng/mL) for 2 h. After 30 min of LPS (10 ng/mL) stimulation (D) cytoplasmic and (E) nuclear proteins were extracted to perform Western-blot using anti-p65 Ab. The membranes were reprobed with anti-GAPDH and Histone Abs for evaluating the loading status of cytoplasmic and nuclear proteins, respectively. The images shown are representatives of 3 independent experiments. *P<0.05 vs. control; # P<0.05 vs. LPS.