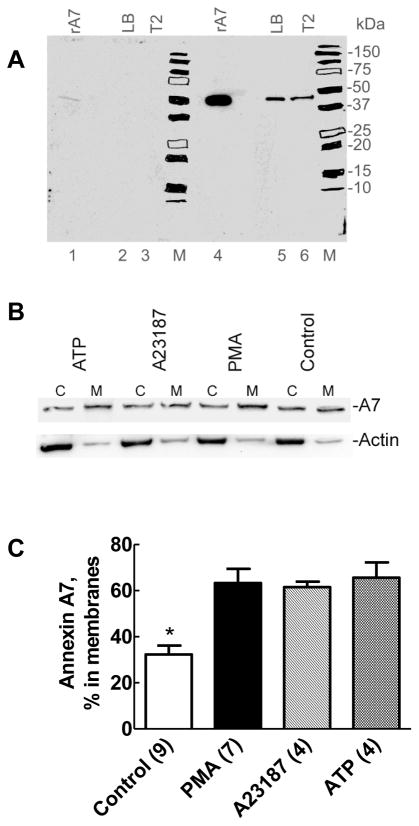
Figure 1. Surfactant secretagogues cause increased membrane-association of endogenous annexin A7.
A. Western blot demonstrates the specificity of antibodies to recombinant rat annexin A7. Proteins (10μg) in isolated lung lamellar bodies, lysates of isolated type II cells cultured for 20–22h, and 1μg of recombinant rat annexin A7 (rA7) were probed for annexin A7 using antibodies preincubated for overnight without (panel containing lanes 4–6 and M) or with (panel with lanes 1–3 and M) 10μg of recombinant rat annexin A7 (rA7). Chemiluminiscence was recorded by autoradiography on an X-ray film with both panels exposed simultaneously. The blot demonstrates the presence of annexin A7 in lamellar bodies and isolated type II cells. Preincubation of antibodies with excess antigen decreased the signal intensity in each lane. M – Molecular weight markers with each position highlighted with a marker pen. B. Isolated rat alveolar type II cells were incubated for 30min without or with ATP (1mM), A23187 (250nM) or PMA (80nM). All of the recovered cytosol (c) and the membrane (M) fractions were probed for annexin A7. The membranes were stripped and probed for actin. C. Densitometry results of western blots for the membranes and cytosol fractions from several experiments showing increased membrane-association of annexin A7 in cells treated for 30min with all three agents used, in comparison to the controls. For these experiments, equal proportions of the cytosol and membrane fractions were probed for annexin A7 levels by western blot analysis followed by densitometry analysis to determine relative distribution of annexin A7. Only membrane-associated A7 levels are shown in each case for better clarity. Each secretagogue increased membrane-association of annexin A7. Results are expressed as mean ± SE of number of cell preparations shown in parentheses. * P < 0.05 – % distribution in control membranes was different in comparison to all others.