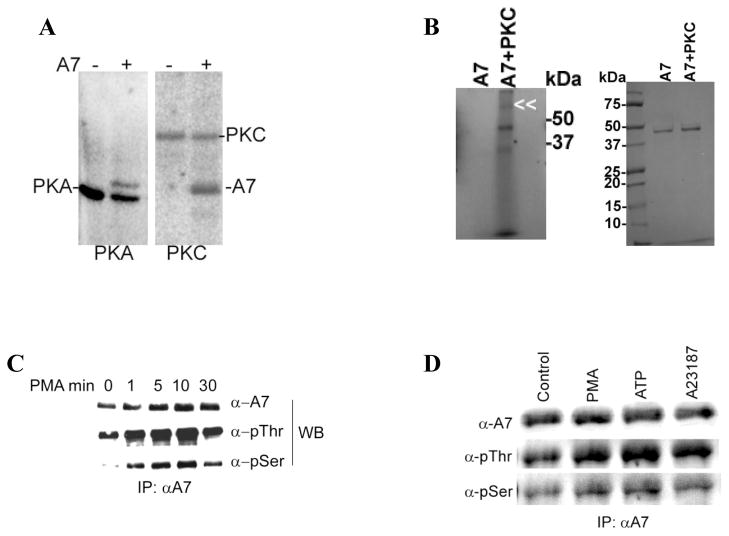
Figure 5. Annexin A7 phosphorylation in vitro and in vivo in alveolar type II cells.
A. Phosphorylation of purified recombinant rat annexin A7 (rA7) by commercially available catalytic subunit of PKA and by purified rat brain PKC. Purified A7 (1μg) was incubated with ATP-γ-32P in presence of required co-factors for 1h. The proteins were separated by SDS-PAGE, stained to localize A7, and imaged with a phosphor-imager to determine phosphorylation. Both the kinase enzyme and rA7 were phosphorylated. B. Phosphorylation of purified A7 in the absence or presence of PKC. Purified A7 was incubated in the described assay mixture in the absence or presence of PKC and in the presence of ATP-γ-32P. Left panel shows phosphorylation of A7 as determined by imaging of radioactivity using a phosphorimager. The phosphorylation of PKC is indicated by double arrowhead. A7 position is just below the 50kDa maker. Right panel shows Coomassie Blue-stained gels showing comparable amounts of A7 in two lanes. Results show no authophosphorylation of A7 under these conditions. C. Type II cells were incubated for indicated periods without or with 100nM PMA and the immuno-precipitates (IPs) were obtained with A7 antibodies. Western blots of IPs with indicated antibodies showed a time-dependent increase in A7 phosphorylation at the serine and threonine residues. D. Type II cells were incubated for 30min without or with indicated agents and harvested in lysis buffer. Equal amounts of cell lysate proteins were immunoprecipitated using A7 antibodies. Western blots of IPs with A7, p-thr and p-ser antibodies showed equal levels of A7 but increased reactivity with p-ser and p-thr antibodies in stimulated cells in comparison to the controls.