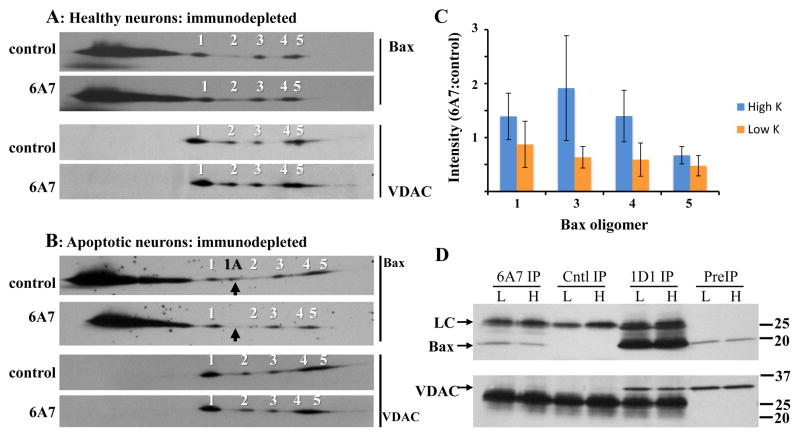
Figure 6. Analysis of Bax and VDAC1 oligomers following 6A7 Bax immunodepletion.
Fourteen million neurons maintained in high (A) or low (B) K for 5h were solubilized with 700μl 1% digitonin. Protein concentrations in the high K control and 1D1 extracts were 540 and 502 μg/ml, respectively, and in the low K control and 1D1 extracts were 400 and 502 μg/ml, respectively. Prior to immunoprecipitation, the samples were concentrated to final protein concentrations of 1253, 1211, 964, and 1203 μg/ml, respectively. The extracts were immunodepleted of active Bax overnight with the 6A7 antibody, or with an irrelevant mouse monoclonal antibody as control (top image of each pair). Aliquots (25μg protein) were subjected to BN SDS-PAGE, and immunoblotted for Bax and VDAC1 using the 1D1 and N18 antibodies, respectively. Images are from one of three independent experiments. The 6A7 antibody depleted the low K extracts of virtually all 1A oligomer, as indicated with the black arrows. (C) Quantification of Bax oligomers 1, 3, 4, and 5 in high and low K 6A7 depleted extracts; oligomer 2 was excluded due to inconsistent detection. Oligomer levels were normalized to the corresponding controls. Statistical analysis by 2-way ANOVA (treatment [high, low K] and oligomer as main effects) yielded a significant effect of treatment (P=0.049) but not of oligomer (P=0.489), with no significant treatment-oligomer interaction (P=0.694). Data are mean ± SEM of three experiments. (D) VDAC1 was undetectable in both high and low K Bax 6A7 immunoprecipitates, but was readily identified in parallel Bax 1D1 samples. H: high K; L: low K; PreIP: initial digitonin extract; Cntl IP: control immunoprecipitation; 6A7 IP: Bax 6A7 immunoprecipitation; 1D1 IP: Bax 1D1 immunoprecipitation; LC: antibody light chain.