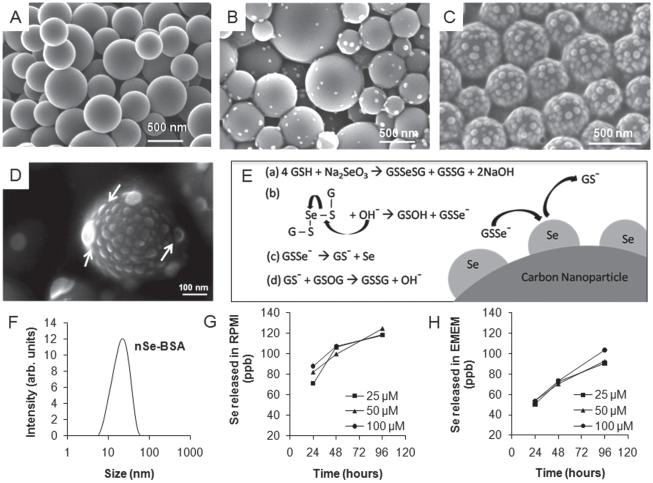
Figure 1.
Synthesis and characterization of selenium/carbon bifunctional nanoparticles. (A) as-produced liquid-crystal-derived carbon nanoparticles synthesized by spray pyrolysis of 0.5-wt% indanthrone disulfonate;[19] (B,C,D) selenium-carbon bifunctional composite nanoparticles with 8.6-wt% Se loading (B) and ~28-wt% Se loading (C); and (D) high-magnifi cation FE-SEM image showing the hemispherical shape of the surface-nucleated nSe clusters. (E) Proposed mechanism: reduction of sodium selenite with glutathione followed by hydroxide ion mediated release of GSSe−1 as a precursor for heterogeneous deposition of self-avoiding elemental selenium nanoclusters; (F) Dynamic light scattering measurement of particle size distribution of nSe stabilized with BSA. (G, H) Release of soluble selenium from nSe over a period of 24, 47 and 96 hours at the doses of 25, 50 and 100 μm in cell culture medium, showing more release in (G) RPMI than (H) EMEM. Note for Se: 1 μM = 78.96 ppb, so only a small fraction of the total Se is mobilized in the extracellular medium after 96 hrs (1–5%).