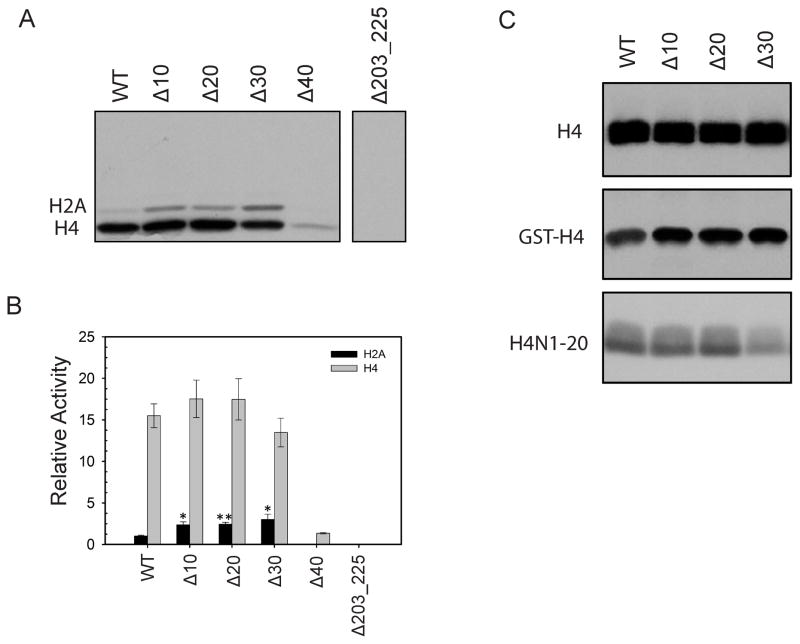
Figure 5. Methyltransferase activities of different AtPRMT10 constructs in vitro.
(A) Indicated AtPRMT10 proteins (5 μg) were used for in vitro methylation activity assay. The reaction mixture was separated on a 15% SDS-PAGE and the autoradiograph of the gel is recorded. The experiments were performed in triplicate and a typical result is shown here. (B) Quantification of the results from Figure 5A. The relative activities presented here were calculated by considering the activity of wild-type AtPRMT10 over H2A as one. The activities of AtPRMT10 mutants over H2A are significantly higher than that of wild-type enzyme (n=3; error bars represent SEM; Student t test *p < 0.03, **p < 0.002). (C) Methyltransferase activities of different AtPRMT10 constructs on H4, GST-H4 and H4N1-20.