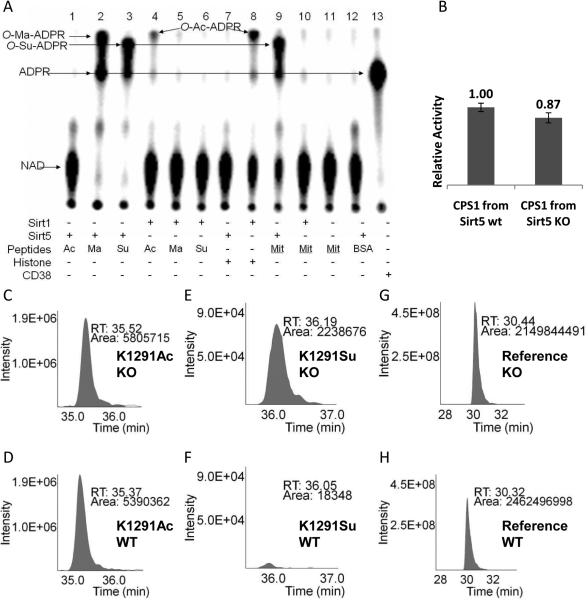
Fig. 3.
(A) Succinyl lysine was detected in bovine liver mitochondria. Sirt5-catalyzed hydrolysis of malonyl and succinyl peptides could be detected using 32P-NAD, which formed 32P-labeled O-Ma-ADPR (lane 2) and O-Su-ADPR (lane 3). No reaction occurred with acetyl peptide (lane 1). The formation of O-Ac-ADPR catalyzed by Sirt1 was detected (lanes 4 and 8). O-Su-ADPR was formed when bovine liver mitochondria peptides were incubated with Sirt5 (lane 9), but not with Sirt1 (lane 10). The control with BSA peptides and Sirt5 did not generate O-Su-ADPR (lane 12). CD38-catalzyed hydrolysis of NAD was used to generate the standard 32P-ADPR spot (lane 13). (B) The CPS1 activities were measured using the liver lysates from Sirt5 wt and KO mice. (n=3, p<0.05). Relative quantitation analysis was achieved by extracted ion chromatograms (XICs) for peak areas of CPS1 K1291 acetyl peptides from Sirt5 KO (C) and wt mice (D); of CPS1 K1291 succinyl peptides from Sirt5 KO (E) and wt mice (F), and of a reference peptide from Sirt5 KO (G) and wt mice (H).