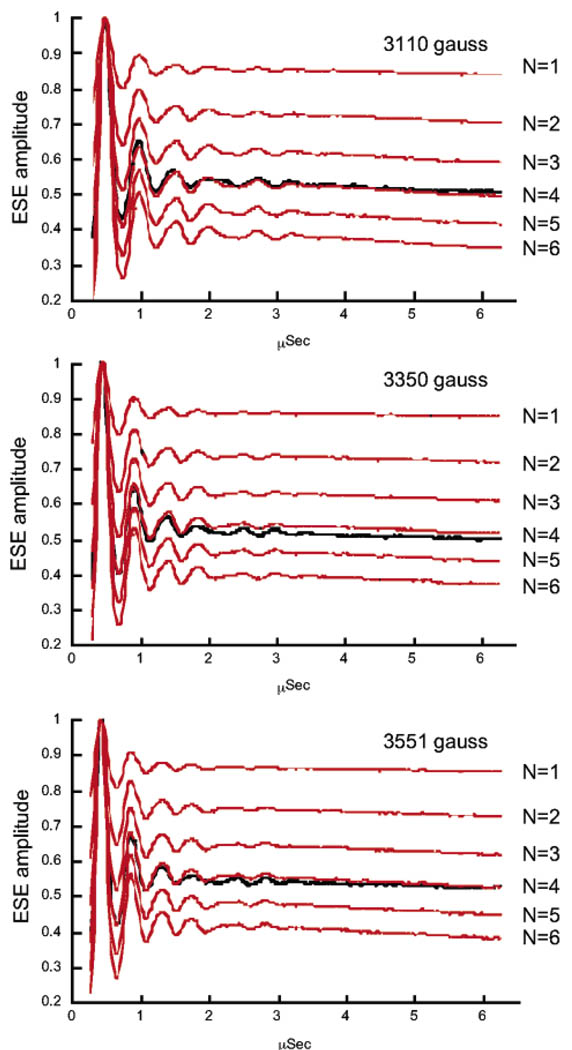
Figure 5.
Hydration level of the high-affinity Mn2+ site in the hammerhead ribozyme determined by 2H ESEEM spectroscopy. 2H three-pulse ESE time-domain traces from deuterium-exchanged HHRz samples, ratioed by data obtained on natural-abundance HHRz samples (see Materials and Methods), are compared with data calculated for hydration levels of N = 1–6 2H2O Mn2+ ligands. Data are plotted as a function of T (µs) (see Materials and Methods) and were obtained at three different magnetic field values with η values (in parentheses) selected for suppression of proton modulation: 3110 G (228 ns); 3350 G (210 ns); 3551 G (198 ns). The average fit to these data gives a value of 4.08 ± 0.12 H2O molecules coordinated to the HHRz-bound Mn2+ ion.