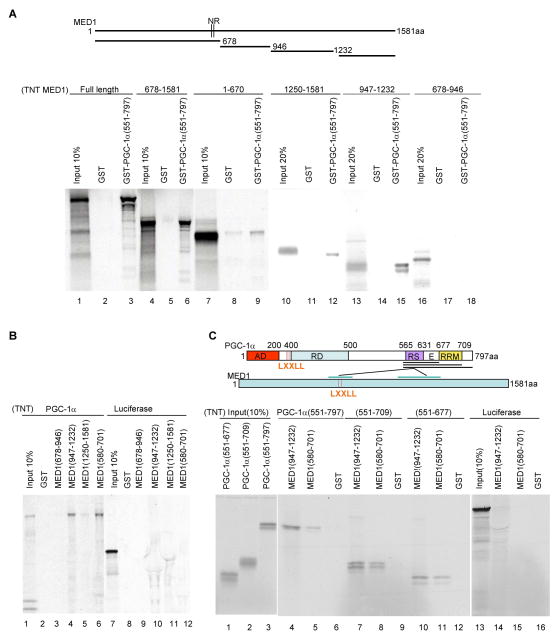
Figure 4. Interactions between PGC-1α domains and MED1 domains.
(A). Interactions of MED1 and MED1 fragments with the PGC-1α C-terminus. Bead-immobilized GST or GST-PGC-1α (551–797) fusion proteins were incubated with in vitro translated, 35S-labeled full length MED1 or MED1 fragments and bound proteins were analyzed by SDS-PAGE and autoradiography. The upper panel depicts MED1 and the derived fragments.
(B) Interactions of MED1 and MED1 fragments with full length PGC-1α. Bead-immobilized GST or indicated GST-fusion proteins were incubated with in vitro translated, 35S-labeled PGC-1α (lanes 1–6) or a luciferase control (lanes 7–12) and bound proteins were analyzed by SDS-PAGE and autoradiography.
(C). Interactions of the PGC-1α RS/E domain with MED1. Bead-immobilized GST, GST-MED1(580–701) or GST-MED1(947–1232) fusion proteins were incubated with the indicated in vitro translated, 35S-labeled PGC-1α fragments (lanes 4–12) or a luciferase control (lanes 13–16) and bound proteins were analyzed by SDS-PAGE and autoradiography. The upper panel summarizes the observed interactions of the PGC-1α RS/E domain with an LXXLL-containing MED1 domain (weaker) and a more C-terminal MED1 domain (stronger).