Abstract
Aging is associated with loss of endurance; however, aging is also associated with decreased fatigue during maximal isometric contractions. The aims of this study were to examine the relationship between age and walking endurance (WE) and maximal isometric fatigue (MIF) and to determine which metabolic/fitness components explain the expected age effects on WE and MIF. Subjects were 96 pre-menopausal women. Oxygen uptake (walking economy) was assessed during a 3-mph walk; aerobic capacity and WE by progressive treadmill test; knee extension strength by isometric contractions, MIF during a 90-s isometric plantar flexion (muscle metabolism measured by 31P MRS). Age was related to increased walking economy (low VO2, r = −0.19, P < 0.03) and muscle metabolic economy (force/ATP, 0.34, P = 0.01), and reduced MIF (−0.26, P < 0.03). However, age was associated with reduced WE (−0.28, P < 0.01). Multiple regression showed that muscle metabolic economy explained the age-related decrease in MIF (partial r for MIF and age −0.13, P = 0.35) whereas walking economy did not explain the age-related decrease in WE (partial r for WE and age −0.25, P < 0.02). Inclusion of VO2max and knee endurance strength accounted for the age-related decreased WE (partial r for WE and age = 0.03, P > 0.80). In premenopausal women, age is related to WE and MIF. In addition, these results support the hypothesis that age-related increases in metabolic economy may decrease MIF. However, decreased muscle strength and oxidative capacity are related to WE.
Keywords: Exercise economy, Oxygen uptake, Muscular strength, Function
Introduction
Resistance to muscle fatigue (typically defined as the loss of force or power in response to intense muscle contraction) is an important factor in maintaining high quality of life and independent function as we age. Currently, there is no consensus as to what effects age has on resistance to fatigue. When fatigue is expressed as a reduction in force during an attempt to maintain maximal force, healthy older adults generally fatigue more slowly than younger adults (Russ et al. 2008; Narici et al. 1991; Lanza et al. 2004; Kent-Braun et al. 2002; Ditor and Hicks 2000; Chung et al. 2007; Callahan et al. 2009) even though age is associated with lower maximal strength and reduced muscle metabolic capacity (Kent-Braun and Ng 2000; Hunter et al. 2002). However, this is not a universal finding. Some studies show no difference in fatigability (Lindstrom et al. 1997; McNeil and Rice 2007) while others have actually showed a reduced resistance to fatigue in older adults compared to younger adults (McNeil and Rice 2007; Baudry et al. 2007; Petrella et al. 2005). Most studies that have not shown older adults to be fatigue resistant have used velocity-dependent exercise: either fixed velocity isokinetic (McNeil and Rice 2007; Baudry et al. 2007), or an explosive power task (Petrella et al. 2005). The studies that show older adults to be more fatigue resistant than younger subjects used maximal isometric contractions (Russ et al. 2008; Kent-Braun et al. 2002; Ditor and Hicks 2000; Chung et al. 2007; Callahan et al. 2009), with the exception of one which used isokinetic contractions (Lanza et al. 2004). Most of the interest in changes in fatigue resistance and endurance capacity with age have been in older, usually more than 55 years, adults even though independent of physical activity, losses in muscle metabolic capacity and walking endurance occur with age even in premenopausal women (Hunter et al. 2002). In addition, increased age in premenopausal women is associated with increased difficulty in performing daily activities such as walking, carrying a box of groceries, and climbing stairs. Finally, age is related to decreased free-living physical activity and increased weight gain in premenopausal women (Hunter et al. 2004). It is therefore, important to study what affects increasing age has on endurance and muscle fatigue in premenopausal women.
Improved fatigue resistance in older adults may be caused by an age-related improvement in muscle metabolic economy (ME) (Kent-Braun 2009; Tevald et al. 2009; Lanza et al. 2005; Hepple et al. 2004). Type II muscle fiber atrophy is a consistent finding in old versus young adults (Bamman et al. 2003; Proctor et al. 1995) while some have also shown a preferential loss of type II muscle fibers (Jacobsson et al. 1990)—both of which lead to a higher percentage area of type I muscle. Several studies have shown that type I muscle fibers are metabolically more economical than type II muscle fibers in humans (Hunter et al. 2001; Coyle et al. 1992), human skinned skeletal muscle fibers (Szentesi et al. 2001) and isolated mouse skeletal muscle (Crow and Kushmerick 1982). Therefore, it is not surprising that a recent study has shown that older adults have higher ME than young (Kent-Braun 2009).
Even though understanding the dynamics of muscular fatigue in older adults is important, it may be more important to understand the factors that are responsible for fatigue in activities that older adults undertake in everyday life. We have previously shown that, independent of physical activity level (as measured with doubly labeled water), age is independently related to reduced treadmill endurance (time to exhaustion on a progressive treadmill test), maximum oxygen uptake (VO2max), muscle metabolic capacity (31P MRS determined recovery rate of ADP), anaerobic glycolysis, creatine kinase activity, and a number of key anaerobic and aerobic muscle enzymes (Hunter et al. 2002). Other investigators have also shown that VO2max and endurance is reduced in older adults (Hagberg et al. 1985; Makrides et al. 1990). It is not clear whether the age-related reduction in endurance capacity in activities such as walking is mediated primarily by a reduction in aerobic capacity or if other factors contribute to the decline.
We have also previously shown that, independent of aerobic capacity, quadriceps isometric muscular strength is related to endurance during a treadmill test lasting over 15 min (Larew et al. 2003). Additionally, several studies have shown that increases in strength may play a role in improving exercise economy, ease in doing activities of daily living, and endurance performance (Hartman et al. 2007; Parker et al. 1996; Frontera et al. 1990; Hickson et al. 1988; Stone et al. 1983). It is well documented that age is associated with reductions in skeletal muscle mass and muscular strength (Hunter et al. 2004). Logic would dictate that part of the reduction in endurance performance with age may be mediated by reductions in muscular strength as well as aerobic capacity. To our knowledge, previous studies have not evaluated these diametric relationships in the same group of subjects or attempted to determine other factors that may be contributing to the age-related increase in fatigue resistance seen in maximal isometric contractions as well as the age-related decrease in locomotor endurance performance. Therefore, the primary purposes of this paper were to examine the relationship between age and locomotor endurance capacity and maximal isometric fatigue resistance, and to determine which metabolic/fitness variables explain the expected age effects on muscular fatigue and locomotor endurance in premenopausal women. Since differences in VO2max between African American (AA) and European American (EA) women have previously been reported (McCarthy et al. 2006), race will be considered as a potential confounder in the analyses.
Methods
Forty-nine AA and 47 EA normal-weight, healthy premenopausal women (22–48 years old) participated in this study. None were taking any medication known to affect body composition, energy expenditure, or exercise performance. The study was approved by the University of Alabama at Birmingham (UAB) Institutional Review Board. All volunteers were screened and briefed about the experimental protocol, and written informed consent was obtained prior to testing.
Women were weight stable for 1 month prior to testing which included a macronutrient-controlled diet for the 2 weeks preceding testing. All testing was performed in the follicular phase of the menstrual cycle (within 10 days of the start of menses). Exercise sessions on the treadmill and in-magnet isometric exercise bouts during 31P MRS acquisitions were separated by at least 2 days
Dual-energy X-ray absorptiometry (DXA)
Body fat percentage was determined by DXA (DPX-L, Lunar Radiation Corp., Madison, WI, USA). The scans were analyzed using the Adult Software (Version 1.33).
Walking endurance and VO2max
In the morning and after an overnight fast, maximum oxygen uptake (VO2max) was determined by indirect calorimetry on a treadmill using a modified Bruce Protocol. After a 3-mph, 2.5% grade warm-up for 4 min, grade was increased 2.5% each minute until voluntary exhaustion. Volume of O2 and CO2 was measured continuously by open-circuit spirometry and analyzed using a Sensormedics metabolic measurement cart (model 2900, Yorba Linda, CA, USA). Heart rate was monitored by a Polar Vantage XL heart rate monitor (Polar Beat, Port Washington, NY, USA). The highest VO2, respiratory exchange ratio (RER), and heart rate achieved within the last 2 min of exercise were recorded as VO2max, RER max, and heart rate max, respectively. Standard criteria for heart rate, respiratory quotient, and plateauing were used to ensure achievement of VO2max (Holly 1988). Treadmill endurance was defined as the time it took to reach exhaustion on the progressive treadmill test (i.e. time after the 4 min warm-up phase).
Submaximal oxygen uptake
Prior to the maximal treadmill test, submaximal oxygen uptake (VO2) was obtained in the steady-state conditions, during the third and fourth minutes of a 4-min 3 mph walk on the treadmill using the Sensormedics metabolic measurement cart.
Strength measures
Knee extension strength was measured isometrically using methods previously described (Hunter et al. 1995). Forces were measured using a universal shear beam load cell (LCC 500; Omega Engineering, Stamford, CT, USA). Force was measured at 70° knee extension on the right lower leg at the level of the lateral malleolus. Subjects were restrained across the upper legs and hips with padded straps. Maximal isometric plantar flexion was measured in a specially made bench that could fit within the magnet. After three trials, three maximal isometric contractions were recorded with 60-s rest between each trial. Test retest reliability for these tests in our lab has a coefficient of variation of <4%.
31P MRS
1H-magnetic resonance images (MRI) and 31P-MRS were collected on the right calf muscle using a 4.1 T whole body imaging and spectroscopy system. Subjects were studied on two separate days. A series of resting calf muscle MRIs were collected on the first day to measure maximal cross-sectional area of the gastrocnemius and soleus muscles. The images were collected using a torroid coil with the following protocol: repetition time (TR) = 1,000 ms, echo time = 14.5 ms, 256-mm field-of-view, and 5 mm slice thickness with a slice separation of 10 mm. The cross-sectional area of the gastrocnemius and soleus muscle group was determined by manually drawing the area around both muscles from the MRIs of each slice. The coefficient of variation (CV) for maximal cross-sectional area in our lab is 3.9% (McCarthy et al. 2006). The maximal cross-sectional area was subsequently used to normalize force output between individuals. In addition, the subjects practiced performing isometric plantar flexion. The CV for maximal isometric plantar flexion in our lab is 4.9% (Larson-Meyer et al. 2000).
On the second day women performed 90-s unilateral, maximal voluntary isometric plantar flexion contractions. A 7-cm 1H/31P surface coil, fastened to the underbelly of the calf muscle, was used to collect 2-s time-resolved 31P-MRS data during 60 s of rest, 90 s of exercise, and 7.5 min of recovery. The 31P-MRS data were collected using a repetition time of 2,000 ms, four dummy pulses, one signal average, and a half-passage adiabatic excitation pulse. This adiabatic pulse produced a uniform 90-degree excitation pulse over the sensitive volume of the coil and increased the signal–noise-ratio of our acquisitions. An example of the 31P MRS data collected with these parameters in our laboratory has previously been published (Larson-Meyer et al. 2000). Peak areas and positions of the phosphate metabolites were found by time domain fitting, using Fit-masters (Phillips Medical Systems, Inc., Shelton, CT, USA) as previously described (Boska 1994). The exercise bench and force collection devices in our laboratory are also described elsewhere (Larson-Meyer et al. 2000).
31P MRS was commonly used to measure the intracellular concentrations of PCr, inorganic phosphate (PI), and ATP. The intracellular pH was also calculated from the chemical shift difference between PCr and Pi. These pieces of information were used to quantitatively study the energetics of skeletal muscle during exercise and recovery (Boska 1994; Newcomer and Boska 1997). A detailed description of the methods and model used for calculating ATP production rates from time-resolved 31P MR spectra has been previously published (Newcomer and Boska 1997). Briefly, PCr’s rate of depletion during exercise was used to measure the ATP production rate from the creatine kinase (CK) reaction (Boska 1994; Newcomer and Boska 1997). AMP concentration was calculated from the adenylate kinase equilibrium (Newcomer and Boska 1997), and ADP concentration was calculated from the equilibrium equation of the CK reaction (Larson-Meyer et al. 2000). The rate of ATP production from anaerobic glycolysis (ANGLY) was calculated from the time courses of pH, PCr, and Pi by assuming a H+ stoichiometry of the ATP producing reactions and a buffering capacity of muscle (Newcomer and Boska 1997). The rate of PCr increase during the first 14 s of recovery was used to estimate the ATP production rate from oxidative phosphorylation (OXPHOS) (Newcomer and Boska 1997; Quistorff et al. 1992). Muscle metabolic economy (ME) was calculated as average force across the last 14 s of force production divided by ATP production rate from the sum of the creatine kinase reaction, ANGLY, and OXPHOS. The sum of these three production rates was defined as the total ATP production rate in this study. As previously reported (Newcomer et al. 2001), the test–retest R2 was 0.88 for ANGLY, 0.87 for the CK reaction, 0.95 for OXPHOS, and 0.99 for ME for 45% plantar flexion tested twice in seven subjects.
Muscle biopsies and Type I fiber type distribution
Percutaneous muscle biopsies were obtained from the lateral head of the gastrocnemius muscle on a subset of 22 subjects using a 5-mm Bergstrom biopsy needle with suction applied via a 60-cc syringe, as we have detailed elsewhere (Hunter et al. 2001). Muscle was immediately mounted on cork with Tissue-Tek O.C.T. mounting medium, oriented cross-sectionally using a dissecting microscope, and frozen in liquid nitrogen-cooled isopentane.
Muscle blocks were sectioned (10 μm) in a cryostat microtome cooled to −22°C. Myofiber distribution was classified by metachromatic dye-ATPase histochemistry (Hunter et al. 2001). Metachromasia was revealed by 0.1% toluidine blue after acid pre-incubation (pH 4.4) and incubation in 0.15% ATP disodium salt (pH 9.4). Type I myofibers were colored turquoise. Type II myofiber subtypes ranged in color from light gray (Type IIa) to violet (Type IIx).
Statistics
Independent t tests were used to determine differences between AA and EA women for all variables. Correlations were obtained for age, race, treadmill endurance, and muscle fatigue versus all other study variables. Based on the results of the simple correlations, linear multiple linear regression was used to evaluate the independent effects of age, exercise economy, and muscle aerobic and anaerobic capacity. Since VO2max and treadmill endurance time were different between the two races, indicating that race may be a potential confounder in modeling treadmill endurance time, race was also included as an adjusting variable in model 2 for treadmill endurance.
Results
Table 1 contains the means and standard deviations for all study variables. No significant differences were observed between AA and EA women with the exception of VO2max and treadmill endurance. In both measures EA women had higher values than AA women.
Table 1.
Means and standard deviations for all study variables
| African American |
European American |
P | |
|---|---|---|---|
| Age (years) | 33.6 ± 5.5 | 34.2 ± 6.3 | 0.61 |
| Height (cm) | 164.0 ± 7.9 | 165.0 ± 5.5 | 0.49 |
| Total body mass (kg) | 62.8 ± 7.3 | 64.2 ± 5.1 | 0.27 |
| BMI (kg/m2) | 23.3 ± 1.5 | 23.6 ± 1.1 | 0.28 |
| Body fat % | 32.2 ± 5.4 | 32.6 ± 4.3 | 0.69 |
| Knee extension strength (kg) | 36.6 ± 14.7 | 41.0 ± 12.6 | 0.12 |
| VO2max (ml/kg/min) | 29.5 ± 4.8 | 33.7 ± 5.5 | <0.01 |
| VO2 for 3 mph walk (ml/kg/min) | 11.6 ± 2.3 | 12.3 ± 1.6 | 0.08 |
| Treadmill endurance (min) | 5.82 ± 1.09 | 6.59 ± 1.37 | <0.01 |
| OxPhos rate (mmol ATP/L/s) | 0.24 ± 0.09 | 0.30 ± 0.10 | 0.07 |
| AnGly rate (mmol ATP/L/s) | 0.129 ± 0.074 | 0.173 ± 0.079 | <0.05 |
| CK rate (mmol ATP/L/s) | 0.068 ± 0.050 | 0.072 ± 0.034 | 0.67 |
| ME (N cm2 mmol ATP/L) | 22.1 ± 8.8 | 19.8 ± 0.6.9 | 0.29 |
| Max force (N/cm2) | 13.9 ± 2.4 | 14.1 ± 2.5 | 0.71 |
| End force (N/cm2) | 9.8 ± 3.0 | 10.0 ± 2.5 | 0.73 |
| Muscle fatigue (N/cm2) | 4.1 ± 2.0 | 4.1 ± 1.8 | 0.71 |
P shows independent t test probability for differences between African American (n = 49 for all variables except 31P MRS variables for which n = 21) and European American (n = 47 for all variables except 31P MRS variables for which n = 33) women
Table 2 contains Pearson Product correlations for race, age, treadmill endurance and muscle fatigue with demographic, fitness, and muscle metabolic data. Age was significantly related to decreased treadmill endurance (r = −0.28, P < 0.01) but increased resistance to fatigue during a maximal isometric contraction (−0.26, P < 0.03). Age was also significantly related to all other variables with the exception of race and CK rate. Race (coded 0 = EA and 1 = AA) was negatively related to knee extension strength, VO2max, Walk VO2 (low VO2 indicating increased economy while walking), ANGLY rate, and treadmill endurance but was not significantly related to CK rate, OXPHOS rate (r = 0.25, P < 0.07), ME, or muscle fatigue. Treadmill endurance was significantly related to race, knee extension strength, OXPHOS rate, and ME but was not significantly related to Walk VO2 (r = 0.15, P = 0.07), ANGLY rate, CK rate, or muscle fatigue. In simple correlations muscle fatigue was only significantly related to age (r = −0.26), walk VO2 (r = 0.37, P < 0.01) and ME (r = −0.43, P < 0.01). Multiple regression analyses were used to determine the effect of exercise economy on the negative simple correlations of treadmill endurance and age (decreased treadmill endurance with age) and muscle fatigue and age (more fatigue resistance with age). The first model (Table 3) shows that after adjusting for Walk VO2, age remains significantly related to treadmill endurance (partial r = −0.25, P < 0.02). However, when muscle fatigue is modeled using age and ME as dependent variables (Table 3), the significant relationship between age and muscle fatigue disappears (partial r = −0.13, P = 0.35), suggesting that the age-related increase in muscle fatigue resistance is primarily the result of improved ME in older individuals. On the other hand, other factors must be playing a major role in decreased treadmill endurance with age. In order to determine whether aerobic fitness and/or muscular strength are responsible for the reduced treadmill endurance with age relationship, another multiple regression was used to estimate treadmill endurance with race (important as a potential confounder), VO2max, and knee extension strength added to the model (Table 4). Age ceases to be an independent partial correlate with treadmill endurance while Walk VO2, VO2max, and knee extension strength all remained significant independent correlates of treadmill endurance. Since muscle metabolic capacity may have been a confounder in explaining the disappearance of the significant muscle fatigue with age relationship, a final multiple regression was conducted with muscle fatigue as the dependent variable and OXPHOS rate, CK rate, ANGLY rate, and maximum isometric plantar flexion strength as independent variables. All variables were significant independent correlates with muscle fatigue except age.
Table 2.
Correlations between variables of interest and age, race, treadmill endurance, and muscle fatigue
| Age | Race | Treadmill endurance |
Muscle fatigue |
|
|---|---|---|---|---|
| Age | −0.28 (<0.01) | −0.26 (<0.03) | ||
| Race | 0.05 (0.30) | −0.30 (<0.01) | 0.01 (0.99) | |
| VO2max | −0.31 (<0.01) | −0.31 (<0.01) | 0.84 (<0.01) | 0.18 (0.18) |
| VO2 for 3 mph Walk | −0.19 (0.03) | −0.19 (0.03) | 0.15 (0.07) | 0.37 (<0.01) |
| Knee extension strength | −0.32 (<0.01) | −0.32 (<0.01) | 0.30 (<0.01) | 0.13 (0.35) |
| Plantar flexion strength | −0.25 (0.05) | −0.07 (0.60) | 0.11 (0.43) | 0.27 (<0.05) |
| OxPhos rate | −0.31 (0.01) | −0.25 (<0.07) | 0.39 (<0.01) | 0.12 (0.40) |
| AnGly rate | −0.29 (<0.02) | −0.27 (<0.05) | 0.19 (0.17) | 0.02 (0.89) |
| CK rate | −0.17 (<0.11) | −0.06 (0.67) | 0.15 (0.28) | −0.05 (0.70) |
| ME | 0.34 (<0.01) | 0.15 (0.29) | −0.38 (0.01) | −0.43 (<0.01) |
P values are given in parenthesis ME metabolic economy
Table 3.
Multiple regression models designed to determine the independent relationships between age and either treadmill endurance or muscle fatigue after adjusting for economy
| Beta | Partial r | P | |
|---|---|---|---|
| Model 1 for walking endurance | |||
| Model R = 0.29 | <0.02 | ||
| Intercept | 7.3180 | <0.01 | |
| VO2 for 3 mph Walk (ml/kg/min) | 0.0645 | 0.10 | 0.32 |
| Age (years) | −0.0558 | −0.25 | <0.02 |
| Model 1 for muscle fatigue | |||
| Model R = 0.46 | <0.01 | ||
| Intercept | 7.3400 | <0.01 | |
| Muscle metabolic economy (N cm2 mmol ATP/L) |
−0.0975 | −0.39 | <0.01 |
| Age (years) | −0.0365 | −0.13 | 0.35 |
Table 4.
Multiple regression models designed to determine the independent relationships between age and either treadmill endurance or muscle fatigue after adjusting for economy and maximal aerobic and anaerobic capacity
| Beta | Partial r | P | |
|---|---|---|---|
| Model 2 for treadmill endurance | |||
| Model R = 0.86 | <0.01 | ||
| Intercept | 0.0819 | 0.93 | |
| Age (years) | 0.0037 | 0.03 | 0.80 |
| Race (0 = EA, 1 = AA) | 0.0823 | 0.06 | 0.57 |
| VO2max (ml/kg/min) | 0.2000 | 0.82 | <0.01 |
| Knee extension strength (kg) | 0.0144 | 0.27 | <0.01 |
| VO2 for 3 mph Walk (ml/kg/min) | −0.0770 | −0.22 | <0.04 |
| Model 2 for muscle fatigue | |||
| Model R = 0.75 | <0.01 | ||
| Intercept | 7.7850 | <0.01 | |
| Age | −0.0158 | −0.07 | 0.63 |
| OxPhos rate (mmol ATP/L/s) | −8.7670 | −0.46 | <0.01 |
| CK rate (mmol ATP/L/s) | −10.8910 | −0.32 | <0.03 |
| AnGly rate (mmol ATP/L/s) | −7.8670 | −0.38 | <0.01 |
| Maximum isometric plantar flexion strength (N/cm2) |
0.4550 | 0.59 | <0.01 |
| ME (N cm2 mmol ATP/L) | −0.2470 | −0.70 | <0.01 |
Since a potential cause for an age-related increase in ME economy could be an age-related increase in metabolically efficient Type I muscle fibers, type I muscle fiber distribution (mean 48.7 ± 12%) was correlated with age, (r = 0.53, P < 0.01) in a sub-sample of 22 subjects (Fig. 1).
Fig. 1.
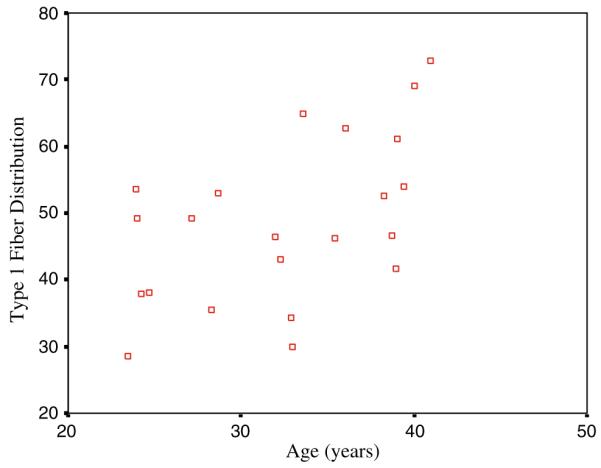
Type I muscle fiber distribution (mean 48.7 12%) correlated with age, (r = 0.53, P < 0.01) in a sub-sample of 22 subjects
Discussion
The results of this study with premenopausal women are consistent with previous studies of older adults which showed that age is related to decreased walking endurance (Hunter et al. 2002; Hagberg et al. 1985; Makrides et al. 1990) but increased resistance to maximal isometric muscle fatigue (Russ et al. 2008; Narici et al. 1991; Lanza et al. 2004; Kent-Braun et al. 2002; Ditor and Hicks 2000; Chung et al. 2007; Callahan et al. 2009). We found that improved ME with age accounts for the age-related improved maximal isometric muscle fatigue (Table 3), but does not account for the decreased walking endurance with increasing age. However, adjustment for VO2max and quadriceps strength accounts for the negative relationship between age and walking economy suggesting that age-related decreases in aerobic fitness and strength mediate the age-related loss in walking endurance in this group of premenopausal women. The overall strength of model 2 for walking endurance (Table 4) in which the coefficient of determination indicates that 75% of the variance in walking economy was accounted for by these variables further supports this contention.
These results are consistent with the hypothesis proposed by Kent-Braun (Kent-Braun 2009) that improved exercise economy with increasing age allows for improved fatigue resistance in tasks that challenge the capacity to exert force, i.e. maximal isometric contractions or force outputs at a very high percentage of maximal capacity. However, age-related loss of muscle strength (Petrella et al. 2005; Hunter et al. 2002), muscle metabolic capacity (Hunter et al. 2002), and total body aerobic capacity (Hunter et al. 2002) may increase the difficulty of completing weight-bearing tasks that require a fixed power output, i.e. activities of daily living such as standing from a chair or walking (Petrella et al. 2005; Landers et al. 2001). Since the age and walking endurance relationship disappears when adjusted for VO2max and knee extension strength, it is probable that the age-related decrease in walking endurance is mediated by an age-mediated loss of aerobic capacity, muscular strength, and increased exercise difficulty. It is impossible to determine whether the age-related decrease in function in this relatively young group of subjects (age ranging from 22 to 48 years) is due largely to increased inactivity with age. However, we have previously shown that decreased muscle metabolic capacity is related to age independent of free living energy expenditure measured with doubly labeled water in pre-menopausal women (Hunter et al. 2002), suggesting aging factors other than reduced physical activity may be affecting muscle function even prior to menopause.
The age-related improvement in exercise economy may at least in part be mediated by an age-related loss of type II muscle fibers. Atrophy and necrosis of type II muscle fibers have been shown to occur in older adults (Proctor et al. 1995; Bamman et al. 2003; Lexell 1995) while type II muscle fibers have been shown to be metabolically less economical in force production than type I muscle fibers (Katz et al. 1986; Coyle et al. 1992; Hunter et al. 2001). Age-related shift in muscle fibers is normally not thought to occur until the sixth or seventh decades. However, in a subset of 22 subjects from this data set, we show that type I muscle fiber percentage is significantly related to age (r = 0.53, P < 0.01), suggesting that this shift may begin early at least in the lateral gastrocnemius, the muscle measured in this study.
On the other hand, preferential type II muscle fiber atrophy is probably responsible for an accelerated loss of power with age, since maximal velocity of shortening and power production are higher in type II muscle fibers (Hunter et al. 2004). Although not well understood, this preferential atrophy of type II muscle fibers and thus power production may be involved in the age-related fatigue-induced shift in power with increasing velocities (Lanza et al. 2003). Another factor that may limit power production with age is impaired central drive to the skeletal muscle. While there may not be consensus as to the degree of deficits, some authors have suggested that there is a decreased ability to recruit motor units with age (Klass et al. 2007). This suggests that older adults not only have a reduced capacity to generate power but also have decreased fatigue resistance in explosive movements. Consistent with this hypothesis, Petrella et al. (2005) have shown that velocity of shortening decreases more rapidly during ten maximal velocity knee extensions at 40% of a maximal voluntary contraction in older (mean age 64 years) than younger (mean age 27 years) men and women.
In this group of premenopausal women, increased walking economy (lower VO2 while walking at 3 mph) was related to increasing age, possibly due to a preferential loss of inefficient type II muscle fibers. This should have given the older women in this study an advantage in treadmill endurance (less oxygen and energy cost for walking). However, decreases in muscular strength and aerobic capacity apparently may have overridden any advantage the older women may have obtained from improved walking economy. To this end, however, it is probable that the relation between increasing age and improved muscle economy may not extend to subjects over 55–65 years, an age in which muscle loss is accelerated (Hunter et al. 2004). In fact, walking economy may actually decrease since total fiber number tends to decline in older adults and therefore the whole muscle is capable of less tension development on activation. This would result in less work being accomplished by low-threshold, more efficient fatigue-resistant type I motor units, increasing the need to activate less efficient, fatigable type II motor units. The improvements in locomotor economy following strength-training-induced increases in strength support this idea (Hartman et al. 2007). More direct support can be found from studies documenting significantly reduced walking economy in older (>65 years) compared with younger (<30 years) men and women (Woo et al. 2006) and significant negative correlations between walking economy and age in women aged over 60 years (unpublished data). If this were the case, loss of muscle mass and strength in old age would have a compound effect on decreasing exercise endurance, via both decreased capacity and decreased exercise economy.
Para 25 Recent research suggests that some of the PCr regeneration early in recovery is due to glycolysis (Forbes et al. 2009). In addition, partial PCr recovery was reported during ischemic contractions (Lanza et al. 2006). Taken together, these studies indicate that not all the initial recovery of PCr following exercise is due to oxidative phosphorylation. However, several observations in this study indicate that oxidative phosphorylation predominates during recovery. First, oxidative phosphorylation rate after exercise was significantly related to muscle fatigue even after adjusting for anaerobic glycolysis and creatine kinase rate (Model 2, Table 4) showing an independence from these anaerobic pathways for predicting muscle fatigue, suggesting significant oxidative phosphorylation. Second, substituting ADP time constant, a measure that is reflective of the entire collected time period yielded almost identical results as shown in Model 2 in Table 4.
In conclusion, the results of this study show that fatigue resistance is increased with increasing age, primarily due to improved muscle metabolic economy with age, in premenopausal women. However, reductions in strength and aerobic capacity are related to decreased walking endurance in premenopausal women. These results show that aging process starts prior to menopause in women. When combined with other data on premenopausal women that document losses in the ability to perform daily activities with increasing age (Hunter et al. 2004) it is apparent that even premenopausal women should develop lifestyle strategies such as exercise training and weight control to slow the age-related losses in endurance and function that begin to occur at the age of about 30 years. Further research is needed to determine whether these results are also applicable to men.
Acknowledgments
We acknowledge Bob Petri, David Bryan, Amy Thomas, and Betty Darnell for technical assistance and Paul Zuckerman for Program coordinating. We wish to thank our sources of support: NIH grants R01 DK 49779, R01 DK51684, R01 AG027084-S1, General Clinical Research Center grant M01-RR00032, Clinical Nutrition Research Unit grant P30-DK56336, and UAB University-Wide Clinical Nutrition Research Center grant. The Nestlé Food Co., Solon, OH, USA, and the Stouffer’s Lean Cuisine® provided food entrées. We have no professional relationships with companies or manufacturers who will benefit from the results of the present study.
Contributor Information
Gary R. Hunter, Department of Human Studies, University of Alabama at Birmingham, 901 South 13th Street, Birmingham, AL 35294-1250, USA Department of Nutrition Sciences, University of Alabama at Birmingham, Birmingham, USA.
C. Scott Bickel, Department of Physical Therapy, University of Alabama at Birmingham, Birmingham, USA.
Pedro Del Corral, Department of Nutrition Sciences, University of Alabama at Birmingham, Birmingham, USA.
Nuala M. Byrne, School of Movement Studies, Queensland University of Technology, Brisbane, QLD, Australia
Andrew P. Hills, School of Movement Studies, Queensland University of Technology, Brisbane, QLD, Australia
D. Enette Larson-Meyer, Department of Family and Consumer Sciences, University of Wyoming, Laramie, WY, USA.
Marcas M. Bamman, Department of Physiology and Biophysics, University of Alabama at Birmingham, Birmingham, USA
Bradley R. Newcomer, Nuclear Medicine Technology Department, University of Alabama at Birmingham, Birmingham, USA
References
- Bamman MM, Hill VJ, Adams GR, Haddad F, Wetzstein CJ, Gower BA, Ahmed A, Hunter GR. Gender differences in resistance-training-induced myofiber hypertrophy among older adults. J Gerontol A Biol Sci Med Sci. 2003;58:108–116. doi: 10.1093/gerona/58.2.b108. [DOI] [PubMed] [Google Scholar]
- Baudry S, Klass M, Pasquet B, Duchateau J. Age-related fatigability of the ankle dorsiflexor muscle during concentric and eccentric contractions. Eur J Appl Physiol. 2007;100:515–525. doi: 10.1007/s00421-006-0206-9. [DOI] [PubMed] [Google Scholar]
- Boska M. ATP production rates as a function of force level in the human gastrocnemius/soleus using P MRS. MRM. 1994;32:1–10. doi: 10.1002/mrm.1910320102. [DOI] [PubMed] [Google Scholar]
- Callahan DM, Foulis SA, Kent-Braun JA. Age-related fatigue resistance in the knee extensor muscles is specific to contraction mode. Muscle Nerve. 2009;39:692–702. doi: 10.1002/mus.21278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung LH, Callahan DM, Kent-Braun JA. Age-related resistance to skeletal muscle fatigue is preserved during ischemia. J Appl Physiol. 2007;103:1628–1635. doi: 10.1152/japplphysiol.00320.2007. [DOI] [PubMed] [Google Scholar]
- Coyle EF, Sidossis LS, Horowitz JF, Beltz JD. Cycling efficiency is related to the percentage of type I muscle fibers. Med Sci Sport Exerc. 1992;24:782–788. [PubMed] [Google Scholar]
- Crow MT, Kushmerick MJ. Chemical energetics of slow-and fast-twitch muscle of the mouse. J Gen Physiol. 1982;79:147–166. doi: 10.1085/jgp.79.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ditor DS, Hicks AL. The effect of age and gender on the relative fatigability of the human adductor pollicis muscle. Can J Physiol Pharmacol. 2000;78:781–790. [PubMed] [Google Scholar]
- Forbes SJ, Paganini AT, Slade JM, Towse TF, Meyer RA. Phosphocreatine recovery kinetics following low-and high-intensity exercise in human triceps surae and rat posterior hindlimb muscles. Am J Physiol. 2009;296:161–170. doi: 10.1152/ajpregu.90704.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frontera WR, Meredith CN, O’Reilly KP, Evans WJ. Strength training and determinants of Vo2max in older men. J Appl Physiol. 1990;68:329–333. doi: 10.1152/jappl.1990.68.1.329. [DOI] [PubMed] [Google Scholar]
- Hagberg JM, Allen WK, Seals DR, Hurley BF, Ehsani AA, Holloszy JO. A hemodynamic comparison of young and older endurance athletes during exercise. J Appl Physiol. 1985;58:2041–2046. doi: 10.1152/jappl.1985.58.6.2041. [DOI] [PubMed] [Google Scholar]
- Hartman MJ, Fields DA, Byrne NM, Hunter GR. Resistance training improves metabolic economy during functional tasks in older adults. J Strength Cond Res. 2007;21:91–95. doi: 10.1519/00124278-200702000-00017. [DOI] [PubMed] [Google Scholar]
- Hepple RT, Hagen JL, Krause DJ, Baker DJ. Skeletal muscle aging in F344BN F1-hybrid rats. II. Imprved contractile economy in senescence helps compensate for reduced ATP-generating capacity. J Gerontol Biol Sci. 2004;59A:1111–1119. doi: 10.1093/gerona/59.11.1111. [DOI] [PubMed] [Google Scholar]
- Hickson RC, Dvorak BA, Gorostiaga EM, Kurowski TT, Foster C. Potential for strength and endurance training to amplify endurance performance. J Appl Physiol. 1988;65:2285–2290. doi: 10.1152/jappl.1988.65.5.2285. [DOI] [PubMed] [Google Scholar]
- Holly RG. Measurement of maximal rate of oxygen uptake. In: Blair SN, Painter P, Pate RR, Smith LK, Taylor CB, editors. Resource manual for guidelines for exercise testing and prescription. Lea & Febiger; Philadelphia: 1988. pp. 171–177. [Google Scholar]
- Hunter GR, Treuth MS, Weinsier RL, Kekes-Szabo T, Kell SH, Roth DL, Nicholson C. The effects of strength conditioning on older women’s ability to perform daily tasks. J Am Geriatr Soc. 1995;43:756–760. doi: 10.1111/j.1532-5415.1995.tb07045.x. [DOI] [PubMed] [Google Scholar]
- Hunter GR, Newcomer BR, Larson-Meyer DE, Bamman MM, Weinsier RL. Muscle metabolic economy is inversely related to exercise intensity and type II myofiber distribution. Muscle Nerve. 2001;24:654–661. doi: 10.1002/mus.1051. [DOI] [PubMed] [Google Scholar]
- Hunter GR, Newcomer BR, Weinsier RL, Karapondo DL, Larson-Meyer DE, Joanise DR, Bamman MM. Age is independently related to muscle metabolic capacity in premenopausal women. J Appl Physiol. 2002;93:70–76. doi: 10.1152/japplphysiol.01239.2001. [DOI] [PubMed] [Google Scholar]
- Hunter GR, McCarthy JP, Bamman MM. Effects of resistance training on older adults. Sports Med. 2004;34:329–348. doi: 10.2165/00007256-200434050-00005. [DOI] [PubMed] [Google Scholar]
- Jacobsson A, Borg K, Edstrom L. Fiber-type composition, structure and cytoskeletal protein location of fibers in the anterior tibial muscle. Acta Neuropathol. 1990;80:459–468. doi: 10.1007/BF00294604. [DOI] [PubMed] [Google Scholar]
- Katz A, Sahlin K, Henriksson J. Muscle ATP turnover rate during isometric contraction in humans. J Appl Physiol. 1986;60:1839–1842. doi: 10.1152/jappl.1986.60.6.1839. [DOI] [PubMed] [Google Scholar]
- Kent-Braun JA. Skeletal Muscle fatigue in old age: whose advantage. Exerc Sport Sci Rev. 2009;37:3–9. doi: 10.1097/JES.0b013e318190ea2e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent-Braun JA, Ng AV. Skeletal muscle oxidative capacity in young and older women and men. J Appl Physiol. 2000;89:1072–1078. doi: 10.1152/jappl.2000.89.3.1072. [DOI] [PubMed] [Google Scholar]
- Kent-Braun JA, Ng AV, Doyle JW, Towse TF. Human skeletal muscle responses vary with age and gender during fatigue due to incremental isometric exercise. J Appl Physiol. 2002;93:1813–1823. doi: 10.1152/japplphysiol.00091.2002. [DOI] [PubMed] [Google Scholar]
- Klass M, Baudry S, Duchateau J. Voluntray activation during maximal contraction with advancing age: a brief review. Eur J Appl Physiol. 2007;100:543–551. doi: 10.1007/s00421-006-0205-x. [DOI] [PubMed] [Google Scholar]
- Landers KA, Hunter GR, Wetzstein CJ, Bamman MM, Weinsier RL. The interrelationship among muscle mass, strength, and the ability to perform physical tasks of daily living in younger and older women. J Gerontol. 2001;56A:B443–B448. doi: 10.1093/gerona/56.10.b443. [DOI] [PubMed] [Google Scholar]
- Lanza IR, Towse TF, Caldwell GE, Wigmore DM, Kent-Braun JA. Effects of age on human muscle torque, velocity, and power in two muscle groups. J Appl Physiol. 2003;95:2361–2369. doi: 10.1152/japplphysiol.00724.2002. [DOI] [PubMed] [Google Scholar]
- Lanza IR, Russ DW, Kent-Braun JA. Age-related enhancement of fatigue resistance in evident in men during both isometric and dynamic tasks. J Appl Physiol. 2004;97:967–975. doi: 10.1152/japplphysiol.01351.2003. [DOI] [PubMed] [Google Scholar]
- Lanza IR, Befroy DE, Kent-Braun JA. Age-related changes in ATP-producing pathways in human skeletal muscle in vivo. J Appl Physiol. 2005;99:1736–1744. doi: 10.1152/japplphysiol.00566.2005. [DOI] [PubMed] [Google Scholar]
- Lanza IR, Wigmore DM, Belfroy DE, Kent-Braun JA. In vivo ATP production during free-flow and ischaemic muscle contractions in humans. J Physiol. 2006;577:353–367. doi: 10.1113/jphysiol.2006.114249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larew K, Hunter GR, Larson-Meyer EE, Newcomer BR, McCarthy JP, Weinsier RL. Muscle metabolic function, exercise performance, and weight gain. Med Sci Sports Exerc. 2003;35:230–236. doi: 10.1249/01.MSS.0000048641.47125.1C. [DOI] [PubMed] [Google Scholar]
- Larson-Meyer DE, Newcomer BR, Hunter GR, Hetherington HP, Weinsier R. 31P MRS measurement of mitochondrial function in skeletal muscle: reliability, force-level sensitivity and relation to whole body maximal oxygen uptake. NMR Biomed. 2000;13:14–17. doi: 10.1002/(sici)1099-1492(200002)13:1<14::aid-nbm605>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Lexell J. Human aging, muscle mass, and fiber type composition. J Gerontol A Biol Sci Med Sci. 1995;50(Spec No.):11–1. doi: 10.1093/gerona/50a.special_issue.11. [DOI] [PubMed] [Google Scholar]
- Lindstrom B, Lexell J, Gerdie B, Downham D. Skeletal muscle fatigue and endurance in young and old men and women. J Gerontol A Biol Sci Med Sci. 1997;52:B59–B66. doi: 10.1093/gerona/52a.1.b59. [DOI] [PubMed] [Google Scholar]
- Makrides L, Heigenhauser GJF, Jones NL. High intensity endurance training in 20 to 30 and 60 to 70 year old healthy men. J Appl Physiol. 1990;69:1792–1798. doi: 10.1152/jappl.1990.69.5.1792. [DOI] [PubMed] [Google Scholar]
- McCarthy JP, Hunter GR, Weinsier RL, Larson-Meyer DE, Bamman MM, Landers KA, Newcomer BR. Ethnic differences in triceps surae muscle-tendon complex and walking economy. J Strength Cond Res. 2006;20:511–518. doi: 10.1519/17395.1. [DOI] [PubMed] [Google Scholar]
- McNeil DJ, Rice CL. Fatiguability is increased with age during velocity-depedent contractions of the dorsiflexors. J Gerontol A Biol Sci Med Sci. 2007;62:624–629. doi: 10.1093/gerona/62.6.624. [DOI] [PubMed] [Google Scholar]
- Narici MV, Bordini M, Cerretelli P. Effect of aging on human adductor pollicis muscle function. J Appl Physiol. 1991;71:1277–1281. doi: 10.1152/jappl.1991.71.4.1277. [DOI] [PubMed] [Google Scholar]
- Newcomer BR, Boska M. Adenosine triphosphate production rates, metabolic economy calculations, ph, phosphomonoesters, phosphodiesters, and force output during short-duration maximal isometric plantar flexion exercises and repeated maximal isometric plantar flexion exercises. Muscle Nerve. 1997;20:336–346. doi: 10.1002/(SICI)1097-4598(199703)20:3<336::AID-MUS11>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Newcomer BR, Larson-Meyer DE, Hunter GR, Landers KL, Weinsier RL. Skeletal muscle metabolism in overweight and post-overweight women: an isometric exercise study using 31P magnetic resonance spectroscopy. Int J Obes. 2001;25:1309–1315. doi: 10.1038/sj.ijo.0801673. [DOI] [PubMed] [Google Scholar]
- Parker ND, Hunter GR, Treuth MS, Kekes-Szabo T, Kell SH, Weinsier R, White M. Effects of strength training on cardiovascular responses during a submaximal walk and a weght-loaded walking test in older females. J Cardpulm Rehabil. 1996;16:56–62. doi: 10.1097/00008483-199601000-00007. [DOI] [PubMed] [Google Scholar]
- Petrella JK, Kim J, Tuggle SC, Hall SR, Bamman MM. Age differences in knee extension power, contratile velocity, and fatigability. J Appl Physiol. 2005;98:211–220. doi: 10.1152/japplphysiol.00294.2004. [DOI] [PubMed] [Google Scholar]
- Proctor DN, Sinning WE, Walro JM, Sieck GC, Lemon PWR. Oxidative capacity of human muscle fiber types: effects of age and training status. J Appl Physiol. 1995;78:2033–2038. doi: 10.1152/jappl.1995.78.6.2033. [DOI] [PubMed] [Google Scholar]
- Quistorff B, Johansen L, Sahlin K. Absence of phosphocreatine resynthesis in human calf muscle during ischaemic recovery. Biochemistry. 1992;291:681–686. doi: 10.1042/bj2910681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russ DW, Towse TF, Wigmore DM, Lanza IR, Kent-Braun JA. Contrasting influences of age and sex on muscle fatigue. Med Sci Sports Exerc. 2008;40:234–241. doi: 10.1249/mss.0b013e31815bbb93. [DOI] [PubMed] [Google Scholar]
- Stone MH, Wilson GD, Blessing D, Rozenek R. Cardiovascular responses to short-term olympic style weight training in young men. Can J Appl Sport Sci. 1983;8:134–139. [PubMed] [Google Scholar]
- Szentesi P, Zaremba R, van Mechelen W, Stienen M. ATP utilization for calcium uptake and force production in different types of human skeletal muscle fibres. J Physiol. 2001;531:393–403. doi: 10.1111/j.1469-7793.2001.0393i.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tevald MA, Foulis SA, Lanza IR, Kent-Braun JA. Lower energy cost of skeletal muscle contractions in older humans. Am J Physiol Endocrinol Metab. 2009;298:P729–P739. doi: 10.1152/ajpregu.00713.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo JS, Derleth C, Stratton JR, Levy WC. The influence of age, gender, and training on exercise efficiency. J Am Coll Cardiol. 2006;47:1049–1057. doi: 10.1016/j.jacc.2005.09.066. [DOI] [PubMed] [Google Scholar]


