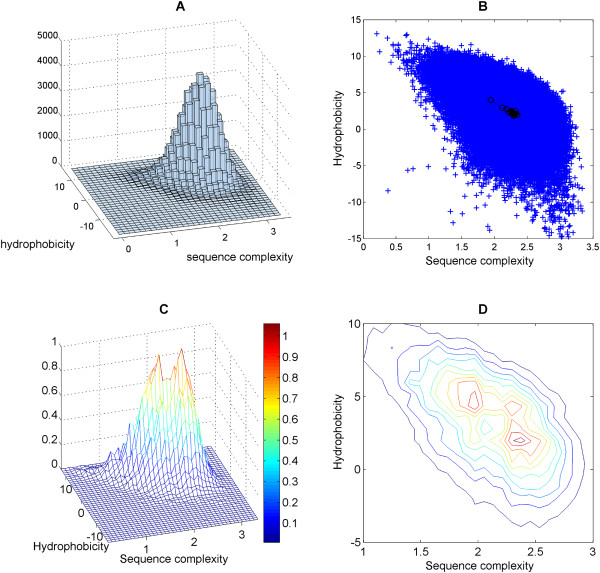
Figure 1.
Distribution of UniProt-derived TM segments in the complexity/hydrophobicity plot. 181132 transmembrane helices from single and multi-spanning membrane proteins were extracted based on the keyword FT_TRANSMEM described in the UniProt annotation file (dated 16-09-2010). The three-dimensional histogram (Figure 1A) shows a fin-shaped distribution across the diagonals of the complexity and hydrophobicity space. The cross-section of the histogram (Figure 1B) shows a tear-shaped pattern. Furthermore, the medians of 15 sets of spanning membrane proteins (containing 1 to 15 TM helices respectively) were computed and denoted by the black circles. The medians were connected in ascending order, starting from the single-spanning set. The trace of the complexity and hydrophobicity from the single-spanning set to the four-TM spanning set indicates a progressive shift towards high complexity and low hydrophobicity. The medians converge to almost a singular cloud beyond the five-transmembrane-spanning set. The three-dimensional surface plot (Figure 1C) shows the hybrid distributions of the single-spanning and those with at least five membrane-spanning TM helices. The single-spanning ones appear more 'simple' while, as a trend, the others seem more 'complex'. The two to four-spanning helices are excluded as they were considered to be 'in-betweeners'. Two distinct peaks (in red) can be seen in the surface plot, denoting 'simple' and 'complex' populations respectively. The contour plot (Figure 1D) emphasizes on the two peaks.