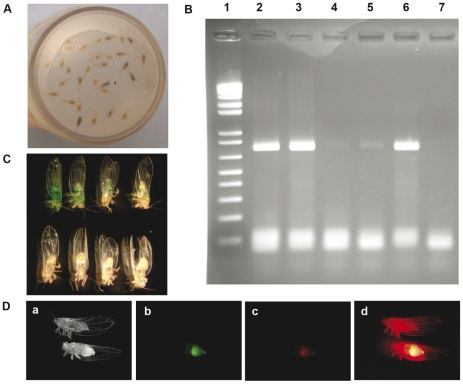
Figure 3. Validation of oral ingestion for B. cockerelli.
(A) Psyllid feeding system. Teneral pale-colored adult psyllids are placed in plastic vials (25mm X 45mm). The feeding solution is 15% sucrose (w: v) sandwiched between two sheets of stretched parafilm. Plastic vials containing the psyllids were maintained at room temperature in the dark. (B) Psyllids were fed on 15% sucrose containing 50ng/µL of GFP PCR product. After 48h feeding, psyllids were collected, DNA extracted and analyzed by PCR. Lane 1, 1Kb plus DNA Marker; Lane 2 and 3, 0.004ng and 0.0004 ng GFP PCR product only as a positive control for PCR reaction. Lanes 4 and 5, PCR products from GFP DNA-fed psyllids; Lane 6, PCR product from sucrose-fed psyllid extract mixed with 0.004 ng GFP DNA; Lane 7, sucrose-fed psyllid used as negative control. (C) 1∶10 (v:v) dilution of green coloring dye was added to 15% sucrose and used for feeding. After 24h the psyllids were examined by low power light microscopy. Upper row is food coloring-fed psyllids and the lower row shows sucrose-fed psyllid controls. (D) 50 ng/µL Cy™3-labeled GFP dsRNA was supplied in 15% sucrose and fed for psyllids. After 48h feeding, the fluorescence was visualized by fluorescence microscopy (Leica DM5000B) equipped with a mercury lamp (ebq100, ISOLATED). In each panel, the lower psyllid was fed with Cy™3-labeled dsRNA and the upper psyllid was sucrose-fed psyllid control. Psyllids were visualized under white light (a) or under fluorescent light of different excitations at 460nm, 480nm and 436nm (b-d).