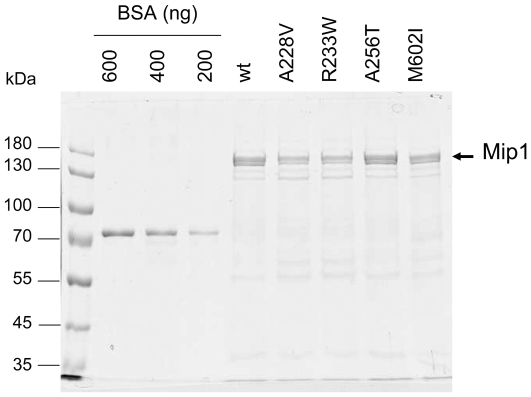
Figure 3. SDS-PAGE analysis of wild-type and mutant Mip1 purified by phosphocellulose chromatography.
Mip1 proteins were purified through DEAE and phosphocellulose chromatographies as reported in Materials and methods. Proteins (5 µl of the phosphocellulose fraction) were subjected to 7% SDS-PAGE and detected by colloidal Coomassie blue staining of the gel. The concentration of Mip1 was determined based on the signal intensity given by known concentrations of bovine serum albumin (BSA) (Pierce). Quantification was performed using the Kodak Image Station 4000 R software. Based on the results of several SDS-PAGE experiments, Mip1 concentration was found to be on average 600 ng for wild-type and Mip1-A256T, 350 ng for Mip1-A228V and Mip1-R233W, and 300 ng for Mip1-M602I. Mip1 was detected as a double band, resulting from partial C-terminal truncation of Mip1. This proteolysis has no incidence on Mip1 activities.