Abstract
Objective
To investigate the efficacy of extracranial-intracranial (EC-IC) bypass surgery using a radial artery interposition graft (RAIG) for surgical management of cerebrovascular diseases.
Methods
The study involved a retrospective analysis of 13 patients who underwent EC-IC bypass surgery using RAIG at a single neurosurgical institute between 2003 and 2009. The diseases comprised intracranial aneurysm (n=10), carotid artery occlusive disease (n=2), and delayed stenosis in the donor superficial temporal artery (STA) following previous STA-middle cerebral artery bypass surgery (n=1). Patients were followed clinically and radiographically.
Results
Bypass surgery was successful in all patients. At a mean follow-up of 53.4 months, the short-term patency rate was 100%, and the long-term rate was 92.3%. Twelve patients had an excellent clinical outcome of Glasgow Outcome Scale (GOS) 5, and one case had GOS 3. Procedure-related complications were a temporary dysthesia on the graft harvest hand (n=1) and a hematoma at the graft harvest site (n=1), and these were treated successfully with no permanent sequelae. In one case, spasm occurred which was relieved with the introduction of mechanical dilators.
Conclusion
EC-IC bypass using a RAIG appears to be an effective treatment for a variety of cerebrovascular diseases requiring proximal occlusion or trapping of the parent artery.
Keywords: EC-IC arterial bypass, Radial artery interposition graft, Revascularization
INTRODUCTION
Extracranial-intracranial (EC-IC) bypass surgery is being increasingly used in the surgical management of cerebrovascular diseases, especially for the treatment of complex, unclippable aneurysms and occlusive cerebrovascular disease20-22). EC-IC bypass surgery using a saphenous vein interposition graft (SVIG) has been the preferred treatment for giant aneurysms of the clinoid or cavernous internal carotid artery (ICA) where acute sacrifice of the ICA is often required. The advantages of using the saphenous vein (SV) for grafting are that a long length can be harvested, and it is readily available, also it is a high-flow conduit17,29). However, the disadvantages are that there is often a large diameter disparity between the donor vessel and the SVIG, the SV is vulnerable to traumatic injury during harvesting, scarring can be significant following harvesting of long graft lengths, and the SV has a lower resistance to kinking or torsion compared to arterial grafts3,23).
The use of radial artery interposition graft (RAIG) has not been broadly accepted because of its tendency to cause spasm and occlusion when used in coronary bypass surgery7,8). However, interest in the use of RAIG for cerebral revascularization has been rekindled with the advent of the pressure distention technique described by Sekhar et al.25,26) and drug therapy to prevent graft spasm. A number of studies have reported satisfactory long-term patency rates for RAIG25,26).
The present study evaluated clinical outcomes in 13 patients with cerebrovascular diseases who underwent EC-IC bypass surgery using a RAIG. The pressure distention technique was used in all cases.
MATERIALS AND METHODS
Data from 13 patients treated in a single institute between 2003 and 2009 who underwent an EC-IC bypass using a RAIG were retrospectively analyzed (Table 1). Patients comprised 9 females and 4 males, and had a mean age of 57.2 years (range, 44-76). Patients presented with intracranial aneurysm (n=10), carotid artery occlusive disease (n=2), and delayed stenosis in the donor after previous superficial temporal artery-middle cerebral artery (STA-MCA) bypass surgery (n=1).
Table 1.
Clinical characteristics and outcomes
GOS : Glasgow Outcome Scale, RAIG : radial artery interposition graft, SAH : subarachnoid hemorrhage, ICA : internal carotid artery, MCA : middle cerebral artery, STA : superficial temporal artery
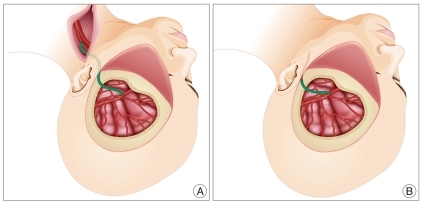
Of the 10 patients treated for acute therapeutic occlusion, six had large to giant aneurysms at the ICA, one had giant thrombosed fusiform aneurysms at the mid-basilar artery, one had an ICA pseudoaneurysm presenting with massive epistaxis, and two had a blood blister-like aneurysm on the dorsal wall of the ICA. Two patients with chronic occlusive cerebrovascular disease presented with ischemic symptoms had a hypoplastic ipsilateral STA for use as a donor vessel for STA-MCA bypass. One patient used RAIG due to delayed stenosis in the donor STA following previous STA-middle MCA bypass surgery. Nine patients underwent long-segment bypass surgery from the proximal external carotid artery (ECA) to the M2 segment of the MCA (Fig. 1A), and 4 patients underwent short-segment bypass from the proximal STA to the M2 segment of the MCA (Fig. 1B).
Fig. 1.
Illustrations depicting long- and short-segment bypassing using a radial artery interposition graft (RAIG). A : Long-segment bypass. One end of the RAIG (green color) is anastomosed to the M2 segment of the middle cerebral artery and the other end is anastomosed the cervical external carotid artery in an end-to-side fashion. B : Short-segment bypass. The RAIG is anastomosed to the proximal superficial temporal artery in an end-to-end fashion at a site just anterior to the tragus.
Patient evaluation
Most patients were evaluated preoperatively using magnetic resonance image, computed tomography (CT) and conventional trans-femoral catheter angiography (TFCA). TFCA was performed in all patients to evaluate the location, shape and size of the aneurysm, and to obtain vital information regarding collateral circulation from other major intracranial vessels. In addition, selective external carotid angiography and selective brachial arteriography of the non-dominant arm were performed to evaluate the diameters and collaterals of the donor and graft vessels. Balloon test occlusion (BTO) of the ICA was performed in the same session to evaluate collateral vascular flow. BTO findings were the main criteria used when deciding upon the appropriate bypass conduit. Single proton emission computed tomography (SPECT) with radioisotope infusion during temporary ICA occlusion was performed to gather additional important information regarding subclinical hypoperfusion. Patients were classified based on the BTO and SPECT data : 1) good clinical tolerance during BTO, no sign of hypoperfusion on SPECT; 2) good clinical tolerance with appearance of some portion of hypoperfusion on SPECT; and 3) poor clinical tolerance. For class 1) patients, the ICA or MCA can simply be occluded without bypass. For class 2) patients, it is routine to perform a low-flow bypass such as STA-MCA bypass. For class 3) patients, it is usual to perform a high-flow bypass such as SVIG (ECA-SVIG-M2) or RAIG (ECA-RAIG-M2)11).
In the patient group with chronic occlusive cerebrovascular diseases, Diamox SPECT has a pivotal role for selecting patients who will benefit from bypass surgery. In our institute, a decrease in perfusion or perfusion reserve in one or more territories on SPECT combined with sustained clinical symptoms after optimal antiplatelet treatment is an indication for an STA-MCA bypass. However, RAIG was employed when the STA could not be used for reasons such as injury during dissection, unsuitable diameter compared to the donor, and previous use in other surgical procedures. When RAIG had too large to bypass the M4 recipient artery that was too small and narrow in size, thus high-flow bypass ECA-RAIG-M2 was performed.
Operation
The long-segment bypass technique (Fig. 1A) has been described in detail elsewhere24,25). Briefly, the patient was positioned in the supine position with the head rotated to the contralateral side of the affected side. The frontotemporal head, ipsilateral neck and non-dominant side of the arm was prepared and draped. A craniotomy centered at the sylvian fissure was performed in the frontotemporal region, and the cervical ICA, ECA and common carotid artery were dissected and prepared for anastomosis. The radial artery was harvested using a linear skin incision just above the artery and "popped" with heparinized saline. The lumen of the radial artery graft was flushed with heparinized saline and both graft ends were prepared for anastomosis. The graft was soaked in heparinized saline. A distal anastomosis was performed first between the RAIG and M2 branches of the MCA in an end-to-side fashion using an interrupted suture of 8-0 or 9-0 nylon. After confirming no leakage, the RAIG was passed along the subcutaneous tunnel under the skin anterior to the tragus. Great care was taken to avoid rotating or kinking of the graft below the skin tunnel, and this was confirmed by temporarily releasing the temporary clip placed at the graft near the MCA and observing a smooth, pulsatile blood flow through the non-anastomosed end of the graft. Another end-to-side anastomosis was performed between the proximal ECA and the RAIG using a continuous 6-0 nylon suture. The proximal ECA rather than the ICA was used as a donor proximal vessel because the ECA is less vulnerable than the ICA to ischemia associated with the long cross-clamping times required for the anastomosis procedure.
Short-segment bypass surgery (Fig. 1B) was performed between the proximal STA and M2 segment of the MCA in the same manner as the long-segment bypass. The RAIG was anastomosed to the proximal STA in an end-to-end manner just anterior to the tragus as that allows for the maximum diameter to be achieved without injuring the facial nerve.
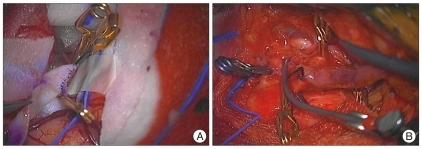
Intraoperative photos showed long-segment bypass from ECA to M2 segment of the MCA using radial artery (Fig. 2).
Fig. 2.
A : Intraoperative photo shows anastomosis from radial artery to M2 segment of the middle cerebral artery in and end-to-side fashion. B : It shows anastomosis from cervical external carotid artery to radial artery in an end-to-side fashion.
All antiplatelet drugs or anticoagulants were discontinued 1 week prior to surgery. Calcium channel blockers were not administered for prevention of RAIG spasm. An intravenous injection of 3,000 U heparin during the bypass was done in the first 4 patients of the series, but not in the subsequent 9 patients. In all patients, intraluminal irrigation was done using heparinized saline. All patients were administered 300 mg aspirin after recovery from anesthesia, followed by ongoing 100 mg oral aspirin per day.
In patients with therapeutic occlusion of the ICA, endovascular occlusion of the parent vessel was performed at the postoperative 4 day to 13, after confirmation of the patency of the bypass graft. Parent vessel occlusion was performed using detachable balloon in 1 case, and using detachable coils in 5 cases. In others, conventional TFCA was performed at postoperative 1 week.
Follow-up involved clinical examinations and neuroimaging studies including TFCA or CTA to verify graft patency. Three dimensional CTA follow-up was performed at postoperative 3 months and 1 year. Clinical outcome was assessed using the Glasgow Outcome Scale (GOS)14).
RESULTS
The overall mean follow-up time was 42 months (range, 8-64). Of the 10 intracranial aneurysm patients, nine had a GOS score of 5 (i.e., excellent clinical outcome) (Table 1). The remaining patient had a GOS score of 3 who had a blister-like aneurysm on the ICA (Table 1, case 3). The majority of patients were in good clinical condition without any signs of regional hypoperfusion. Of the 3 chronic occlusive disease patients, all had GOS scores of 5, and none showed signs of any new ischemic or stroke symptoms.
Graft patency persisted in 12 patients (92.3%). One graft failure was seen. This patient was a 56-year-old female with a blood blister-like aneurysm on the dorsal wall of the supraclinoid ICA where direct clipping of the aneurysm resulted in tearing of the aneurysm neck. As a result, an emergency EC-IC bypass was performed after prolonged ICA trapping. A RAIG was used since the STA was lost during the craniotomy procedure, and postoperative patency was confirmed using angiography. However, postoperative brain swelling resulted in the need for decompressive craniectomy surgery and resultant sacrifice of the graft. The patient presented preoperatively with Hunt and Hess Grade IV due to a ruptured aneurysm, and showed no improvement in this condition postoperatively.
Two patients experienced minor reversible complications related to surgery. The first complication was postoperative hand dysthesia related to the graft harvest. This symptom spontaneously improved and resolved completely within 3 months after surgery. The second complication occurred in a patient with a cavernous ICA aneurysm. At postoperative day 10, the aneurysm was treated with endovascular occlusion of the affected ICA after confirming graft patency. After the procedure, a large hematoma developed at the RAIG donor site in the left forearm, possibly due to intravenous heparinization during the procedure. The hematoma was successfully treated by squeezing it through a small incision along the previous incision, and applying compression dressings for several days.
Case 1
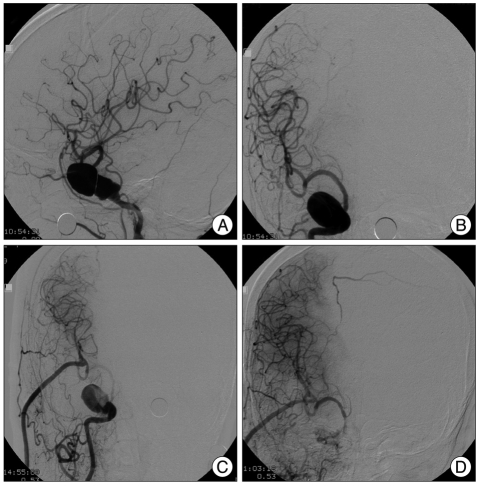
A 61-year-old woman presented with complaints of prolonged repeated headaches (case 1). A 2×4 cm giant cavernous aneurysm was diagnosed (Fig. 3A, B). EC-IC bypass using a RAIG was performed. At postoperative 10 days, the proximal ICA portion was occluded using an endovascular method (Fig. 3C, D). The patient was in good condition after the procedure. CT angiography at postoperative 7 months showed a patent graft and no trace of the aneurysm. The patient had no neurologic deficits.
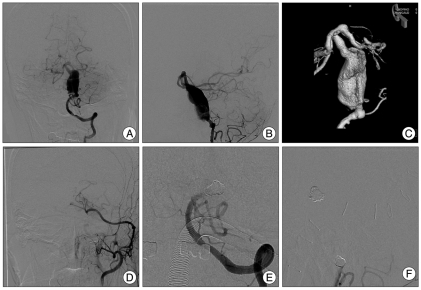
Fig. 3.
A 61-year-old woman presented with a headache, and was found to have a huge aneurysm of the right cavernous internal carotid artery. A and B : Preoperative conventional angiography showing a giant aneurysm measuring 2×4 cm. C : Postoperative angiography revealed good patency of the bypass graft. D : After endovascular occlusion of the proximal ICA, the aneurysm is no longer filled. Angiography shows blood flow through the RAIG supplying the whole middle cerebral territory and the supraclinoid portion of the ICA. ICA : internal carotid artery, RAIG : radial artery interposition graft.
Case 2
A 60-year-old man presented with headache and neck pain, and was found to have a huge dissection aneurysm on basilar artery (Fig. 4). Preoperative conventional angiography showed a giant dissection aneurysm measuring 1.6×3.2 cm (Fig. 4A, B, C). STA-Superior cerebellar artery anastomosis was performed using a RAIG. Postoperative angiography revealed good patency of the bypass graft (Fig. 4D). After endovascular occlusion of the proximal right vertebra artery, the aneurysm was no longer filled. The left vertebral artery also occlusion (it was not demonstrated) (Fig. 3E, F).
Fig. 4.
A 60-year-old man presented with headache and neck pain, and was found to have a huge dissection aneurysm on basilar artery. A, B and C : Preoperative conventional angiography show a giant dissection aneurysm measuring 1.6×3.2 cm. D : Post-bypass surgery angiography reveal good patency of the bypass graft. E and F : After endovascular occlusion of the proximal right vertebra artery, the aneurysm is no longer filled.
DISCUSSION
Why high flow?
Acute occlusion of the ICA either accidentally or intentionally during treatment of ICA aneurysms or tumors results in stroke in 30-37% of patients18,19). EC-IC bypass has been used to avoid ischemic complications after ICA occlusion. An STA-MCA bypass is simple, easy to perform and has a high success rate, and is therefore a common surgical modality for providing compensation for an occluded ICA during cerebrovascular surgery4,15). It also has the advantage of no additional surgical preparation since the donor graft is harvested in the same surgical field and it can be used in an emergency situation if not injured during the opening procedure. However, the STA-MCA bypass is a low-flow conduit (20-40 mL/min) and cannot supply sufficient blood in cases of ICA sacrifice, especially if collaterals such as the anterior communicating or posterior communicating artery provide poor supply. The risk of postoperative ischemia is greater in low-flow than in high-flow bypass grafts. According to the Hagen-Poiseuille equation (Flow=Δ Pressure πr4/8×length×viscosity), a RAIG with a 3 mm diameter provides 81-times the blood flow than an STA with a 1 mm diameter. There is no preoperative test to confirm that the STA can supply sufficient blood to compensate for the occluded ICA10). In addition, the distal short segment of the STA graft near the anastomosis site often narrows, probably due to a change in spasm following dissection and adventitia removal for bypass, and this significantly impairs blood flow through the graft. A RAIG has a larger diameter, and its use even for a short length between the proximal STA and M2 segment of the MCA results in greater bypass blood flow, and decreases the risk of ischemia especially during the vulnerable period to postoperative 4-5 days.
The risk of hemodynamic ischemia is greater early after therapeutic occlusion of the ICA than later since the cerebral perfusion reserve substantially decreases soon after permanent carotid occlusion and then gradually improves over several years27). Therefore, high-flow bypass using a SVIG or RAIG is the most reliable surgical option for preventing ischemic complications after occlusion of the ICA30).
Why the radial artery?
The advantages of saphenous vein graft (SVG) include that they can be long, they are readily available, and they are a high-flow conduit; therefore they have been widely used for ICA reconstruction29). Use of SVIG for the management of giant aneurysms was popularized by Sundt et al.28,29) in the early 1980s and this approach is now widely implemented. Reported SVG patency rates vary from 66-90%, and the rate was shown to improve from 90% to 98% with the use of intraoperative angiography24). A long graft length provides several advantages including preventing tension between the proximal and distal anastomosis sites, allowing for an easier bypass procedure. However, diameter disparity between the donor site and the SVG is a key limitation. Such a disparity may create turbulent flow at the anastomosis site and cause delayed graft occlusion. The radial artery diameter is 3.55±0.45 mm15), which is more compatible than the SV for the recipient M2 portion diameter. Therefore, a RAIG provides a smoother luminal diameter transition from the ECA to the M2 segment than a SVG, and hence less turbulent flow. In addition, the SV is vulnerable to traumatic injury during harvesting. Finally, scarring after SV harvesting is a significant cosmetic concern due to the length, especially if wound complications occurred.
Arterial grafts are generally more resistant to kinking or torsion than vein grafts. Also, arteries do not have valves and remain open even at low flow rates. These factors appear to contribute to the superior long-term patency of RAIG compared to SVG. In terms of harvest site morbidity, RAIG have fewer cosmetic problems and more rapid wound healing than SVG, which promotes earlier postoperative mobilization5). In the present series, hand dysthesia developed in only one case, and this spontaneously resolved by postoperative 3 months. Therefore, careful radial artery dissection and an incision at the distal end maximally 1-2 cm proximal to the radial styloid is required to avoid possible dysthesia at the harvest site.
Patency
A major complication associated with bypass grafting is graft occlusion. Occlusion rates have decreased over time, and are reported to be 5-29%13,16,18). A study by Lawton et al.16) analyzed 61 patients with 63 intracranial complex aneurysms treated with surgery and revascularization. The STA and SV were mainly used, and postoperative angiograms showed that early graft occlusion occurred in 3 patients (5%). Follow-up angiograms in 13 (25%) patients at a mean postoperative 5.1 months found that 1 patient had graft occlusion. The mortality and morbidity rates were 2% and 8%, respectively. A study by Hacein-Bey et al.9) on 9 patients with intracranial aneurysm who underwent STA-MCA or SV bypass grafting reported that postoperative 10 days angiograms showed 1 patient had graft occlusion. Jafar et al.13) reported that of 29 giant aneurysm patients treated using a SVIG, 2 (7%) patients had graft occlusion. Sekhar et al.24-26) reported on 17 intracranial aneurysm patients treated using RAIG between 1994 and 2001. Of the 12 patients treated using the pressure distension technique, none experienced graft occlusion or vasospasm. One (6%) of those 17 patients developed graft occlusion. In the present series, the short-term patency rate was 100%, and the long-term rate was 93%. The cause of the graft occlusion in one case mainly reflected the preoperative surgical plan of direct clipping without preparation for possible bypass surgery in a blood blister-like aneurysm.
Spasm
The radial artery is a muscular artery with a prominent adventitia, and the muscular media of the radial artery has an increased tendency to spasm both in vitro and in the clinical setting. Carpentier and colleagues first proposed the use of the RAIG for coronary artery bypass graft (CABG) in 19736), but reports of spasm and occlusion led to its abandonment. However, other reports detailing long-term angiographically patent radial artery grafts after CABG have revived considerable interest in the use of RAIG1). Ausman et al.2) may have been the first to describe the use of a RAIG for cerebral revascularization, and many Japanese neurosurgeons have reported good surgical results following the use of RAIG in bypass surgery12,15). Improved surgical harvest techniques, the administration of antispasmotic drugs, and development of the pressure distention technique as described by Sekhar and Kalavakonda26) have relieved concerns regarding graft artery spasms. In the present series, perioperative antispasmotic medications were not used, but the pressure distension technique for the RAIG was used in all cases. RAIG spasm occurred in 1 case (case 9), and this involved spasm in a short RAIG segment that resulted in acute occlusion of the graft in the surgical field. The STA-RAIG anastomosis sutures were then removed and Debakey vascular dilators (Aesculap) were introduced into the RAIG to relieve the spasm. The spasm was relieved with serial introduction of larger vascular dilators to the lumen, and blood flow through the bypass from the MCA was achieved. A STA-RAIG anastomosis was performed thereafter.
CONCLUSION
This study indicates that cerebral revascularization using a RAIG is effective for a variety of cerebrovascular diseases. Careful preoperative planning appears essential to prevent surgery related complications. Meticulous undertaking of surgical techniques and careful perioperative management are required to ensure optimal surgical outcomes.
References
- 1.Acar C, Jebara VA, Portoghese M, Beyssen B, Pagny JY, Grare P, et al. Revival of the radial artery for coronary artery bypass grafting. Ann Thorac Surg. 1992;54:652–659. doi: 10.1016/0003-4975(92)91007-v. discussion 659-660. [DOI] [PubMed] [Google Scholar]
- 2.Ausman JI, Nicoloff DM, Chou SN. Posterior fossa revascularization : anastomosis of vertebral artery to PICA with interposed radial artery graft. Surg Neurol. 1978;9:281–286. [PubMed] [Google Scholar]
- 3.Baaj AA, Agazzi S, van Loveren H. Graft selection in cerebral revascularization. Neurosurg Focus. 2009;26:E18. doi: 10.3171/2009.1.FOCUS08303. [DOI] [PubMed] [Google Scholar]
- 4.Boop FA, Story JL, Brown WE, Ansell LV. Cerebral revascularization with an artificial graft : long-term follow-up and discussion of the role of graft pretreatment with modified host endothelial cells. Surg Neurol. 1993;40:155–159. doi: 10.1016/0090-3019(93)90128-n. [DOI] [PubMed] [Google Scholar]
- 5.Buxton B, Fuller J, Gaer J, Liu JJ, Mee J, Sinclair R, et al. The radial artery as a bypass graft. Curr Opin Cardiol. 1996;11:591–598. doi: 10.1097/00001573-199611000-00006. [DOI] [PubMed] [Google Scholar]
- 6.Carpentier A, Guermonprez JL, Deloche A, Frechette C, DuBost C. The aorta-to-coronary radial artery bypass graft. A technique avoiding pathological changes in grafts. Ann Thorac Surg. 1973;16:111–121. doi: 10.1016/s0003-4975(10)65825-0. [DOI] [PubMed] [Google Scholar]
- 7.Chiu CJ. Why do radial artery grafts for aortocoronary bypass fail? A reappraisal. Ann Thorac Surg. 1976;22:520–523. doi: 10.1016/s0003-4975(10)64468-2. [DOI] [PubMed] [Google Scholar]
- 8.Curtis JJ, Stoney WS, Alford WC, Jr, Burrus GR, Thomas CS., Jr Intimal hyperplasia. A cause of radial artery aortocoronary bypass graft failure. Ann Thorac Surg. 1975;20:628–635. doi: 10.1016/s0003-4975(10)65754-2. [DOI] [PubMed] [Google Scholar]
- 9.Hacein-Bey L, Connolly ES, Jr, Duong H, Vang MC, Lazar RM, Marshall RS, et al. Treatment of inoperable carotid aneurysms with endovascular carotid occlusion after extracranial-intracranial bypass surgery. Neurosurgery. 1997;41:1225–1231. doi: 10.1097/00006123-199712000-00001. discussion 1231-1234. [DOI] [PubMed] [Google Scholar]
- 10.Houkin K, Ishikawa T, Kuroda S, Abe H. Vascular reconstruction using interposed small vessels. Neurosurgery. 1998;43:501–505. doi: 10.1097/00006123-199809000-00057. [DOI] [PubMed] [Google Scholar]
- 11.Houkin K, Kamiyama H, Kuroda S, Ishikawa T, Takahashi A, Abe H. Long-term patency of radial artery graft bypass for reconstruction of the internal carotid artery. Technical note. J Neurosurg. 1999;90:786–790. doi: 10.3171/jns.1999.90.4.0786. [DOI] [PubMed] [Google Scholar]
- 12.Ishikawa T, Kamiyama H, Houkin K, Takahashi A, Iwasaki Y, Abe H. Postsurgical observations of mean hemispheric cerebral blood flow with patients receiving high-flow EC-IC bypass using a radial artery graft (preliminary report, one-year observation of 10 hemispheres) Surg Neurol. 1995;43:500–506. doi: 10.1016/0090-3019(95)80098-2. discussion 506-509. [DOI] [PubMed] [Google Scholar]
- 13.Jafar JJ, Russell SM, Woo HH. Treatment of giant intracranial aneurysms with saphenous vein extracranial-to-intracranial bypass grafting : indications, operative technique, and results in 29 patients. Neurosurgery. 2002;51:138–144. doi: 10.1097/00006123-200207000-00021. discussion 144-146. [DOI] [PubMed] [Google Scholar]
- 14.Jennett B, Bond M. Assessment of outcome after severe brain damage. Lancet. 1975;1:480–484. doi: 10.1016/s0140-6736(75)92830-5. [DOI] [PubMed] [Google Scholar]
- 15.Kamiyama H. [Bypass with radial artery graft] No Shinkei Geka. 1994;22:911–924. [PubMed] [Google Scholar]
- 16.Lawton MT, Hamilton MG, Morcos JJ, Spetzler RF. Revascularization and aneurysm surgery : current techniques, indications, and outcome. Neurosurgery. 1996;38:83–92. doi: 10.1097/00006123-199601000-00020. discussion 92-94. [DOI] [PubMed] [Google Scholar]
- 17.Marhold F, Rosen CL. Novel technique to improve vessel mismatch when using saphenous vein bypass grafts for intracranial revascularization procedures. J Neurosurg. 2010;112:1227–1231. doi: 10.3171/2009.9.JNS09367. [DOI] [PubMed] [Google Scholar]
- 18.Miller JD, Jawad K, Jennett B. Safety of carotid ligation and its role in the management of intracranial aneurysms. J Neurol Neurosurg Psychiatry. 1977;40:64–72. doi: 10.1136/jnnp.40.1.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Origitano TC, al-Mefty O, Leonetti JP, DeMonte F, Reichman OH. Vascular considerations and complications in cranial base surgery. Neurosurgery. 1994;35:351–362. doi: 10.1227/00006123-199409000-00001. discussion 362-363. [DOI] [PubMed] [Google Scholar]
- 20.Park EK, Ahn JS, Kwon DH, Kwun BD. Result of extracranial-intracranial bypass surgery in the treatment of complex intracranial aneurysms : outcomes in 15 cases. J Korean Neurosurg Soc. 2008;44:228–233. doi: 10.3340/jkns.2008.44.4.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Patel HC, Teo M, Higgins N, Kirkpatrick PJ. High flow extra-cranial to intra-cranial bypass for complex internal carotid aneurysms. Br J Neurosurg. 2010;24:173–178. doi: 10.3109/02688690903531075. [DOI] [PubMed] [Google Scholar]
- 22.Sanai N, Zador Z, Lawton MT. Bypass surgery for complex brain aneurysms : an assessment of intracranial-intracranial bypass. Neurosurgery. 2009;65:670–683. doi: 10.1227/01.NEU.0000348557.11968.F1. discussion 683. [DOI] [PubMed] [Google Scholar]
- 23.Santoro A, Guidetti G, Dazzi M, Cantore G. Long saphenous-vein grafts for extracranial and intracranial internal carotid aneurysms amenable neither to clipping nor to endovascular treatment. J Neurosurg Sci. 1999;43:237–250. discussion 250-251. [PubMed] [Google Scholar]
- 24.Sekhar LN, Bucur SD, Bank WO, Wright DC. Venous and arterial bypass grafts for difficult tumors, aneurysms, and occlusive vascular lesions : evolution of surgical treatment and improved graft results. Neurosurgery. 1999;44:1207–1223. doi: 10.1097/00006123-199906000-00028. discussion 1223-1224. [DOI] [PubMed] [Google Scholar]
- 25.Sekhar LN, Duff JM, Kalavakonda C, Olding M. Cerebral revascularization using radial artery grafts for the treatment of complex intracranial aneurysms : techniques and outcomes for 17 patients. Neurosurgery. 2001;49:646–658. doi: 10.1097/00006123-200109000-00023. discussion 658-659. [DOI] [PubMed] [Google Scholar]
- 26.Sekhar LN, Kalavakonda C. Cerebral revascularization for aneurysms and tumors. Neurosurgery. 2002;50:321–331. doi: 10.1097/00006123-200202000-00014. [DOI] [PubMed] [Google Scholar]
- 27.Sugawara Y, Kikuchi T, Ueda T, Nishizaki M, Nakata S, Mochizuki T, et al. Usefulness of brain SPECT to evaluate brain tolerance and hemodynamic changes during temporary balloon occlusion test and after permanent carotid occlusion. J Nucl Med. 2002;43:1616–1623. [PubMed] [Google Scholar]
- 28.Sundt TM, 3rd, Sundt TM., Jr Principles of preparation of vein bypass grafts to maximize patency. J Neurosurg. 1987;66:172–180. doi: 10.3171/jns.1987.66.2.0172. [DOI] [PubMed] [Google Scholar]
- 29.Sundt TM, Jr, Piepgras DG, Marsh WR, Fode NC. Saphenous vein bypass grafts for giant aneurysms and intracranial occlusive disease. J Neurosurg. 1986;65:439–450. doi: 10.3171/jns.1986.65.4.0439. [DOI] [PubMed] [Google Scholar]
- 30.Yoshimoto T, Kamiyama H, Abe H, Takikawa S, Ito T. Proximal clipping and bypass between bilateral vertebral arteries using a radial arterial graft for the treatment of a dissecting aneurysm of the vertebral artery. Surg Neurol. 1991;36:476–481. doi: 10.1016/0090-3019(91)90164-5. [DOI] [PubMed] [Google Scholar]