Figure 3. α-ketoglutarate inhibits Enzyme I in vitro.
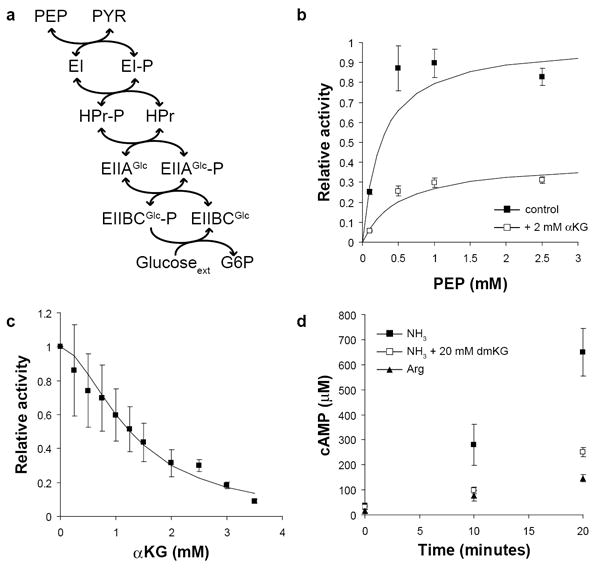
(a) Phosphoenolpyruvate (PEP) donates phosphate, which is transferred in turn to the cytosolic proteins EI, HPr, and EIIAGlc, the membrane transporter EIIBCGlc, and finally glucose, which is simultaneously transported and phosphorylated. All reactions except the final phosphorylation of glucose are reversible. (b) The activity of purified Enzyme I (EI) was determined in the presence (white squares) or absence (black squares) of 2 mM α-ketoglutarate. Plotted values are the estimated enzyme velocities ± standard error, and solid lines are the best fit Michaelis-Menten equation. Values are normalized to the estimated Vmax in the absence of α-ketoglutarate. (c) EI activity was determined in the presence of 1 mM PEP and varying concentrations of α-ketoglutarate. The solid line is the best fit Hill equation for non-competitive inhibition. Values are normalized to the activity with no α-ketoglutarate added. (d) Cyclic-AMP (cAMP) concentration was monitored following a carbon downshift from glucose to glycerol at t = 0. Cells were grown on either 10 mM ammonia or 2.5 mM arginine; where indicated, 20 mM dimethyl-ketoglutarate was present in the glycerol media following downshift. Plotted values are the mean and standard error of three biological replicates.
