Abstract
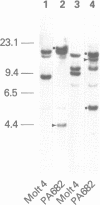
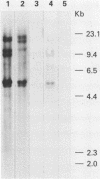
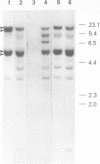
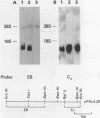
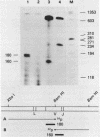
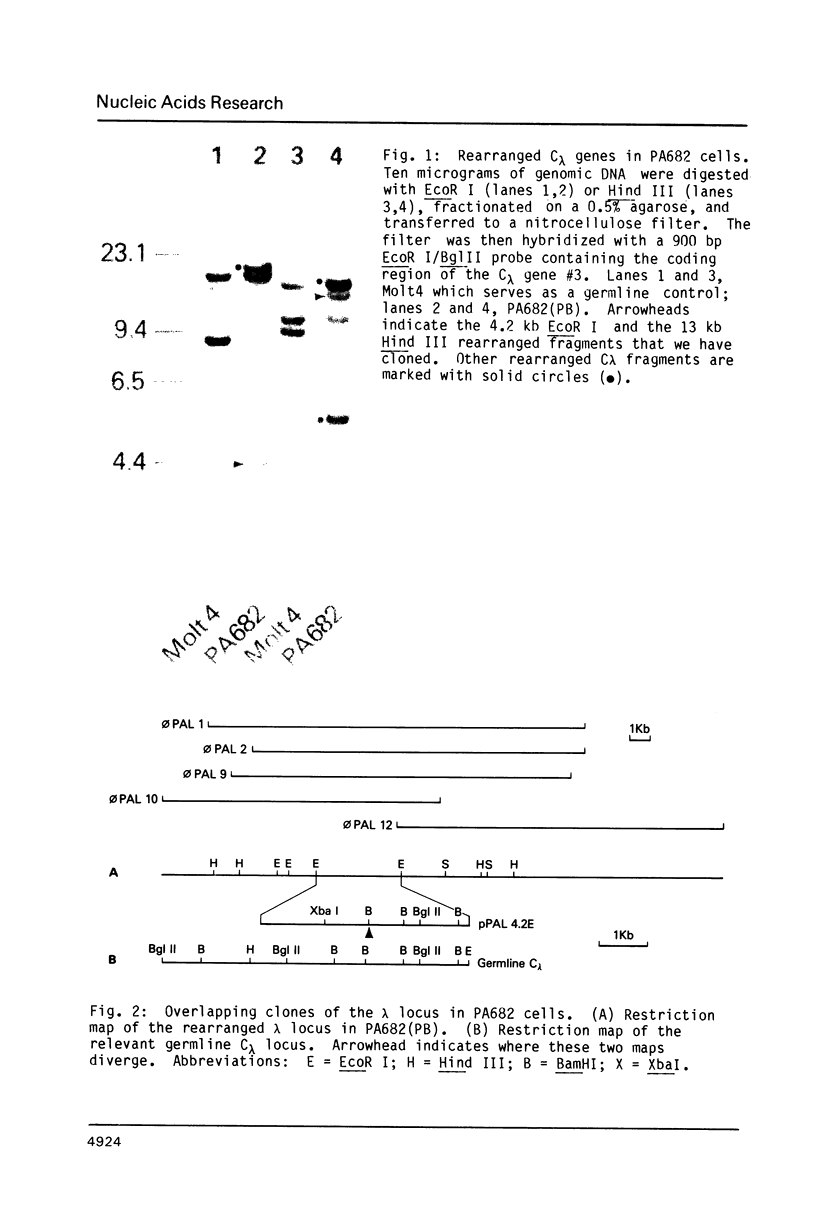
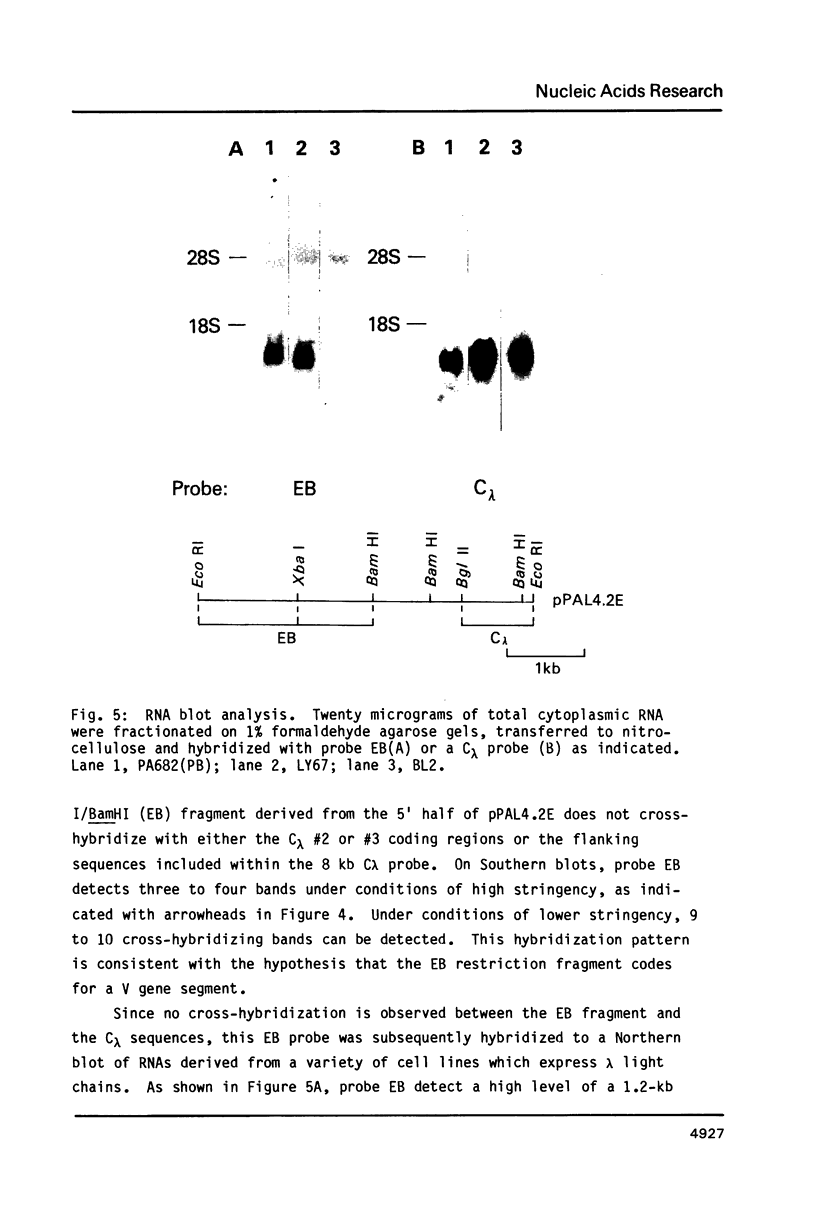
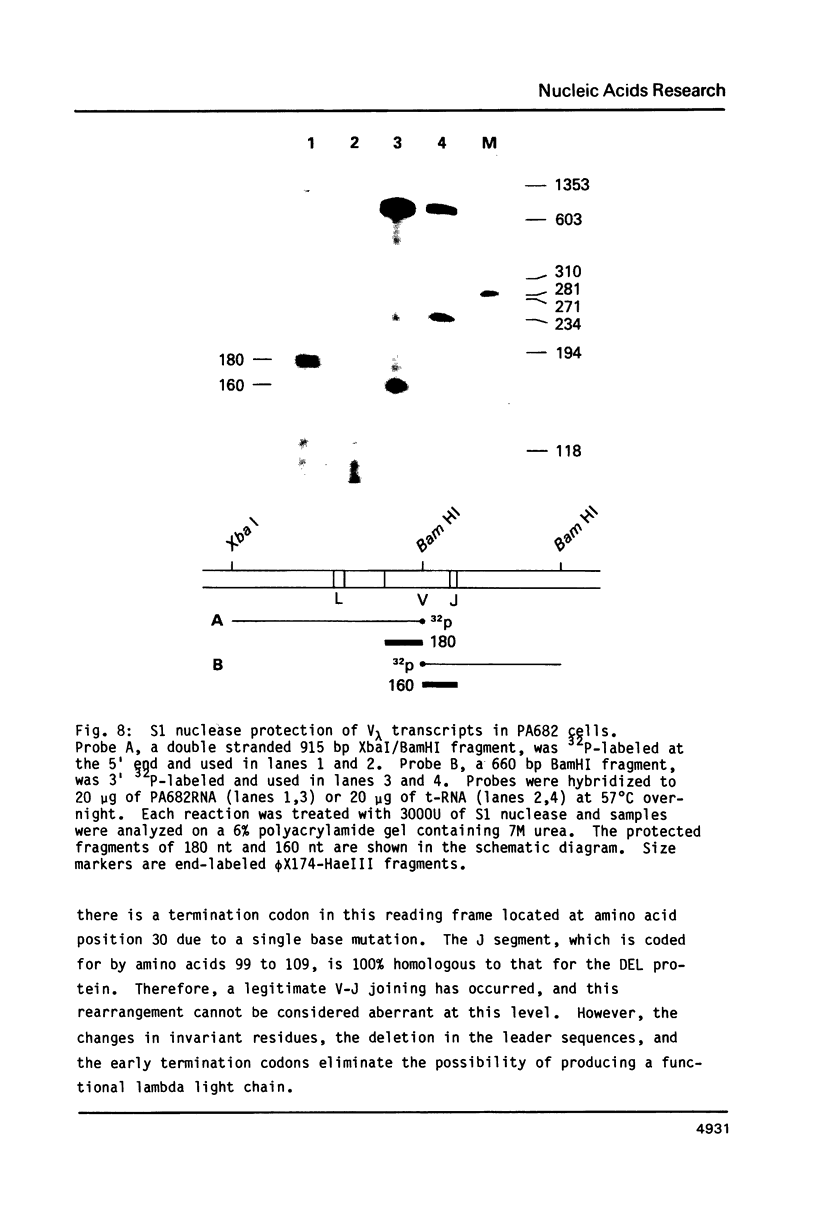
We have cloned and sequenced a rearranged V lambda gene from a Burkitt's lymphoma cell line PA682(PB). This cell line has two rearranged kappa loci and has been shown to be expressing kappa light chains. This V lambda gene has been identified as a member of the V lambda subgroup III gene family based on the homology of the predicted amino acid sequence of PAV lambda with the reported sequences of the V lambda protein DEL of subgroup III. Nine cross-hybridizing bands have been detected on Southern blots and the chromosomal orientation of the V lambda subgroup III gene family has been determined in relation to the V lambda subgroup I gene family. Although the PAV lambda rearrangement has occurred via a legitimate V-J joining and a normal size transcript is detected on Northern blots, the nucleotide sequence reveals a high level of mutations resulting in multiple termination signals within the V gene coding sequence and only a truncated V lambda protein can be translated. This confirms previous observations that although multiple light chain genes may be transcribed, only one functional light chain protein can be synthesized.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alt F. W., Enea V., Bothwell A. L., Baltimore D. Activity of multiple light chain genes in murine myeloma cells producing a single, functional light chain. Cell. 1980 Aug;21(1):1–12. doi: 10.1016/0092-8674(80)90109-9. [DOI] [PubMed] [Google Scholar]
- Anderson M. L., Szajnert M. F., Kaplan J. C., McColl L., Young B. D. The isolation of a human Ig V lambda gene from a recombinant library of chromosome 22 and estimation of its copy number. Nucleic Acids Res. 1984 Sep 11;12(17):6647–6661. doi: 10.1093/nar/12.17.6647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergman Y., Rice D., Grosschedl R., Baltimore D. Two regulatory elements for immunoglobulin kappa light chain gene expression. Proc Natl Acad Sci U S A. 1984 Nov;81(22):7041–7045. doi: 10.1073/pnas.81.22.7041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bothwell A. L., Paskind M., Schwartz R. C., Sonenshein G. E., Gefter M. L., Baltimore D. Dual expression of lambda genes in the MOPC-315 plasmacytoma. Nature. 1981 Mar 5;290(5801):65–67. doi: 10.1038/290065a0. [DOI] [PubMed] [Google Scholar]
- Breathnach R., Chambon P. Organization and expression of eucaryotic split genes coding for proteins. Annu Rev Biochem. 1981;50:349–383. doi: 10.1146/annurev.bi.50.070181.002025. [DOI] [PubMed] [Google Scholar]
- Coleclough C., Perry R. P., Karjalainen K., Weigert M. Aberrant rearrangements contribute significantly to the allelic exclusion of immunoglobulin gene expression. Nature. 1981 Apr 2;290(5805):372–378. doi: 10.1038/290372a0. [DOI] [PubMed] [Google Scholar]
- Dalla-Favera R., Bregni M., Erikson J., Patterson D., Gallo R. C., Croce C. M. Human c-myc onc gene is located on the region of chromosome 8 that is translocated in Burkitt lymphoma cells. Proc Natl Acad Sci U S A. 1982 Dec;79(24):7824–7827. doi: 10.1073/pnas.79.24.7824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eulitz M. A new subgroup of human L-chains of the lambda-type. Primary structure of Bence-Jones protein DEL. Eur J Biochem. 1974 Dec 16;50(1):49–69. doi: 10.1111/j.1432-1033.1974.tb03872.x. [DOI] [PubMed] [Google Scholar]
- Falkner F. G., Zachau H. G. Correct transcription of an immunoglobulin kappa gene requires an upstream fragment containing conserved sequence elements. Nature. 1984 Jul 5;310(5972):71–74. doi: 10.1038/310071a0. [DOI] [PubMed] [Google Scholar]
- Hieter P. A., Hollis G. F., Korsmeyer S. J., Waldmann T. A., Leder P. Clustered arrangement of immunoglobulin lambda constant region genes in man. Nature. 1981 Dec 10;294(5841):536–540. doi: 10.1038/294536a0. [DOI] [PubMed] [Google Scholar]
- Hieter P. A., Korsmeyer S. J., Waldmann T. A., Leder P. Human immunoglobulin kappa light-chain genes are deleted or rearranged in lambda-producing B cells. Nature. 1981 Apr 2;290(5805):368–372. doi: 10.1038/290368a0. [DOI] [PubMed] [Google Scholar]
- Hohn B., Murray K. Packaging recombinant DNA molecules into bacteriophage particles in vitro. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3259–3263. doi: 10.1073/pnas.74.8.3259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korsmeyer S. J., Hieter P. A., Ravetch J. V., Poplack D. G., Waldmann T. A., Leder P. Developmental hierarchy of immunoglobulin gene rearrangements in human leukemic pre-B-cells. Proc Natl Acad Sci U S A. 1981 Nov;78(11):7096–7100. doi: 10.1073/pnas.78.11.7096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magrath I., Erikson J., Whang-Peng J., Sieverts H., Armstrong G., Benjamin D., Triche T., Alabaster O., Croce C. M. Synthesis of kappa light chains by cell lines containing an 8;22 chromosomal translocation derived from a male homosexual with Burkitt's lymphoma. Science. 1983 Dec 9;222(4628):1094–1098. doi: 10.1126/science.6316501. [DOI] [PubMed] [Google Scholar]
- Parslow T. G., Blair D. L., Murphy W. J., Granner D. K. Structure of the 5' ends of immunoglobulin genes: a novel conserved sequence. Proc Natl Acad Sci U S A. 1984 May;81(9):2650–2654. doi: 10.1073/pnas.81.9.2650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott R. W., Frankel F. R. Enrichment of estradiol-receptor complexes in a transcriptionally active fraction of chromatin from MCF-7 cells. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1291–1295. doi: 10.1073/pnas.77.3.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp P. A., Berk A. J., Berget S. M. Transcription maps of adenovirus. Methods Enzymol. 1980;65(1):750–768. doi: 10.1016/s0076-6879(80)65071-x. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Summers J. Physical map of polyoma viral DNA fragments produced by cleavage with a restriction enzyme from Haemophilus aegyptius, endonuclease R-HaeIII. J Virol. 1975 Apr;15(4):946–953. doi: 10.1128/jvi.15.4.946-953.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsujimoto Y., Croce C. M. Molecular cloning of a human immunoglobulin lambda chain variable sequence. Nucleic Acids Res. 1984 Nov 26;12(22):8407–8414. doi: 10.1093/nar/12.22.8407. [DOI] [PMC free article] [PubMed] [Google Scholar]