Figure 1.
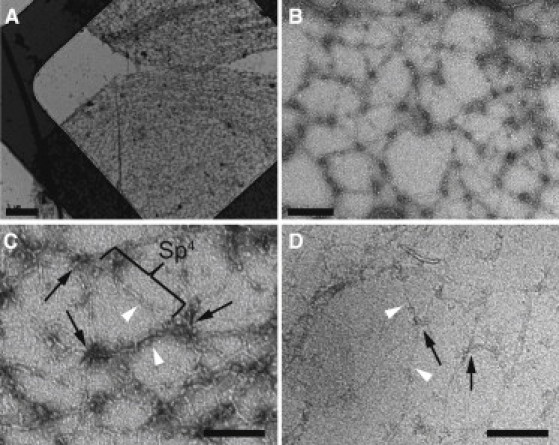
Negative-stain electron microscopy of the isolated, expanded erythrocyte membrane skeletons. (A and B) Low- (A) and medium-magnification (B) electron micrographs of membrane skeletons prepared by Triton X-100 extraction of mouse erythrocyte ghosts, which were adsorbed to carbon-coated grids and negatively stained. As a result of this procedure, the skeletons have been sheared open into a single layer. They have a diameter of ∼10–12 μm, which is about twice as large as the mature mouse erythrocyte, indicating that the skeletons have been stretched. Scale bars, 1 μm (A) and 200 nm (B). (C) Higher-magnification view of the spread meshwork shows junctional complexes (black arrows) that are connected exclusively by spectrin heterotetramers (Sp4) that had an average length of 144 nm (statistics were calculated from four double-tilt tomograms). Scale bar, 120 nm. (D) Cryo-electron micrograph of the expanded membrane skeleton. Skeletons were adsorbed to continuous carbon films and sheared with a jet of buffer before rapid freezing. Junctional complexes (black arrows) and spectrin filaments (white arrowheads) are clearly visible. The dimensions of these features are comparable to those in negatively stained samples. Scale bar, 120 nm.
