Abstract
Pancreatic cancer is the fourth leading cause of cancer-related deaths in this country, and there is currently no effective targeted treatment for this deadly disease. A dire need exists to rapidly translate our molecular understanding of this devastating disease into effective, novel therapeutic options. Mesothelin is a candidate target protein shown by a number of laboratories to be specifically overexpressed in pancreatic cancers and not in the adjacent normal tissue. Translational investigations have shown promising results using this molecule as a therapeutic target (e.g., vaccine strategies). In addition, the mesothelin promoter has been cloned and dissected and can therefore be used as a vehicle for regulating expression of DNA sequences. Using a novel, proven, biodegradable nanoparticulate system, we sought to target mesothelin-expressing pancreatic cancer cells with a potent suicide gene, diphtheria toxin-A (DT-A). We first confirmed reports that a majority of pancreatic cancer cell lines and resected pancreatic ductal adenocarcinoma specimens overexpressed mesothelin at the mRNA and protein levels. High mesothelin-expressing pancreatic cancer cell lines produced more luciferase than cell lines with undetectable mesothelin expression when transfected with a luciferase sequence under the regulation of the mesothelin promoter. We achieved dramatic inhibition of protein translation (>95%) in mesothelin-expressing pancreatic cancer cell lines when DT-A DNA, driven by the mesothelin promoter, was delivered to pancreatic cancer cells. We show that this inhibition effectively targets the death of pancreatic cancer cells that overexpress mesothelin. The work presented here provides evidence that this strategy will work in pre-clinical mouse pancreatic cancer models, and suggests that such a strategy will work in the clinical setting against the majority of pancreatic tumors, most of which overexpress mesothelin.
Keywords: nanoparticles, mesothelin, pancreatic cancer, targeted DNA delivery
Introduction
Pancreatic cancer is generally an incurable and devastating disease. The only therapy with substantive curative success is surgery. However, only 10–20% of patients qualify for surgery.1–3 The molecular events that drive pancreatic tumorigenesis have been well established.4 Current chemotherapeutic strategies include drugs such as 5-fluorouracil and gemcitabine,5 that do not specifically target dysregulated or upregulated molecules in pancreatic cancer. These anti-neoplastic drugs have many off-target side effects when used alone or with radiation therapy. Thus, there is an urgent need to develop novel targeting strategies to treat this disease. One logical therapeutic approach would be to use an upregulated molecular pathway to target a natural toxin to pancreatic cancer cells in a patient.
Mesothelin (MSLN) is a glycosylphosphatidylinositol-linked glycoprotein that was first discovered by Serial Analysis of Gene Expression (SAGE) analysis to be overexpressed in pancreatic ductal adenocarcinoma (PDA).6 Validating their findings, Argani et al. detected MSLN expression in 60 PDA tissue samples, while adjacent normal tissue was negative for MSLN expression.6 Subsequently, using an array of techniques, investigators have validated the reports that MSLN is overexpressed in pancreatic cancer7–15 (Table 1). Further, MSLN has been shown to be overexpressed in a number of other tumor systems.16–18 Recently, encouraging data have emerged from translational and clinical studies attempting to use MSLN as an immunologic target.15,19 Li et al. found MSLN has functional oncogenic properties when overexpressed in the MiaPaCa2 pancreatic cancer cell line.15 For example, forced expression of MSLN significantly increased tumor cell proliferation and migration in vitro, and increased tumor volume by nearly 4-fold in vivo. Similarly, silencing of MSLN inhibited cell proliferation and migration in vitro and halted tumor progression in vivo.15 Hucl et al. recently explored the transcriptional regulation of MSLN and discovered critical sequences necessary for MSLN regulation, including a TEF-1 transcription factor binding site located within a cancer specific enhancing sequence they termed CanScript.14 These studies provide the scientific basis to develop therapeutic strategies for the treatment of pancreatic cancer using regulatory elements of the MSLN gene together with the transcriptional molecular machinery that resides in pancreatic cancer cells.
Table 1.
Mesothelin is overexpressed in pancreatic ductal adenocarcinoma cells and not in normal cells as shown by numerous investigators, techniques and sources
| Author/citation | Technique | Sample sources |
|---|---|---|
| Argani P, Clin Cancer Res 2001,6 | SAGE, in situ hybridization, RT-PCR, Immunohistochemistry | SAGE database, 4 resected PDA, cell lines, 60 primary PDA* |
| Ryu B, Cancer Res 2002,10 | SAGE | 6 pancreatic cancer cell lines compared to controls* |
| Sato N, Cancer Res 2003,12 | Detection of hypomethylation of MSLN | Pancreatic cancer cell lines, resected PDA, xenografts* |
| Iacobuzio-Donahue CA, Cancer Res 2003,9 | 3 techniques: U133 oligonucleotide arrays, cDNA arrays or SAGE | 50 normal samples, 7 samples of chronic pancreatitis, and 39 samples of pancreas cancer tissues or pancreatic cancer cell lines* |
| Sato N, A J Path 2004,11 | RT-PCR or immunohistochemistry | IPMNs (22 invasive and 16 non-invasive)* |
| Watanabe H, Pancreas 20057 | RT-PCR analysis | Pancreatic juice from patients with PDA, IPMN, and chronic pancreatitis |
| Hassan R, A J Clin Path 2005,13 | Immunohistochemistry | 18 cases PDA and chronic pancreatitis |
| Hucl, Cancer Res 2007,14 | Immunohistochemistry, Immunoblotting, RT-PCR | PDA samples and pancreatic cancer cell lines |
| Li M, Mol Can Ther 2008,15 | RT-PCR | PDA* |
Indicates surrounding normal pancreas tissue or other normal pancreas tissue we negative for MSLN expression.
The concept of using highly lethal biological toxins to inhibit cancer cells specifically was put forth over two decades ago when Maxwell and colleagues introduced a plasmid containing the diphtheria toxin-A (DT-A) DNA sequence into cell lines.20 Recently, cationic poly(β-amino ester) biodegradable polymers were used to deliver (DT-A) DNA to prostate cancer cells using the tissue-specific PSA promoter.21–23 These studies showed that the targeted delivery of DT-A to PSA-expressing prostate cancer cells induces apoptosis specifically in these cells. This work laid the foundation for successful local delivery of suicide DT-A DNA sequences to prostate cancer cells in vivo while avoiding residual deleterious effects on surrounding normal tissue.22 These studies also provide a framework in which to test this novel DNA delivery system, combined with tissue/tumor-specific promoter-regulated DNA expression, in other solid tumor systems. In this current study, we extend these previous findings by taking advantage of our knowledge of the regulatory elements that control the expression of MSLN, a molecule that is robustly overexpressed in pancreatic cancer cells (Table 1).
Results
Validation of MSLN mRNA and protein expression in pancreatic cancer cell lines
Multiple studies report overexpression of mesothelin in PDA cells (Table 1). The first step in targeting pancreatic cancer cells was to confirm previous work showing MSLN overexpression in pancreatic cancer cell lines and tissue samples.6,14 MSLN mRNA transcript was detected in CAPAN1 and Hs766T pancreatic cancer cells, but not in PL5 and MiaPaCa2 pancreatic cancer cell lines (Fig. 1A). In another study, we used an antibody to MSLN to immunostain a tissue microarray containing normal pancreatic tissue and primary PDAs. The extent of immunolabeling (0%-negative, 1–25% of cells-focal, >25 of cells-diffuse) and staining intensity (weak, moderate, strong) were recorded for each case. Moderate or strong staining was present in 31 of 40 PDA specimens (representative data, Fig. 1B–D). The staining was focal in five and diffuse in 26 cases. Normal pancreatic tissue showed no staining in eight cases, with faint acinar staining present in two cases, thus confirming previous studies showing MSLN is specifically expressed in pancreatic cancer cells (Fig. 1B–D).
Figure 1.
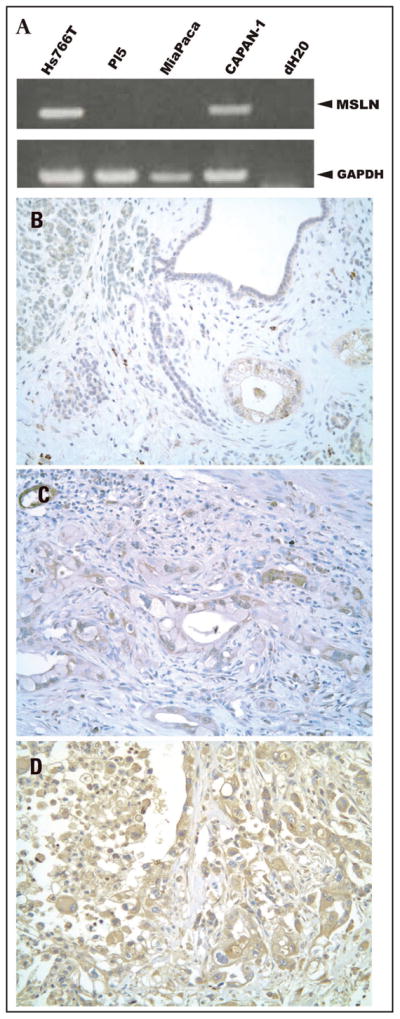
MSLN expression in pancreatic cancer cells. (A) RT-PCR detection of MSLN and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) genes. GAPDH used as a loading control. (B) Moderate cytoplasmic expression of MSLN in well differentiated PDA with no staining or only faint cytoplasmic MSLN expression present in normal ducts and acini. (C) Moderate MSLN cytoplasmic staining in moderately differentiated PDA. (D) Strong MSLN expression in poorly differentiated PDA. (Magnification: 200X).
Nanoparticle-delivered MSLN promoter is active specifically in MSLN+ pancreatic cancer cells
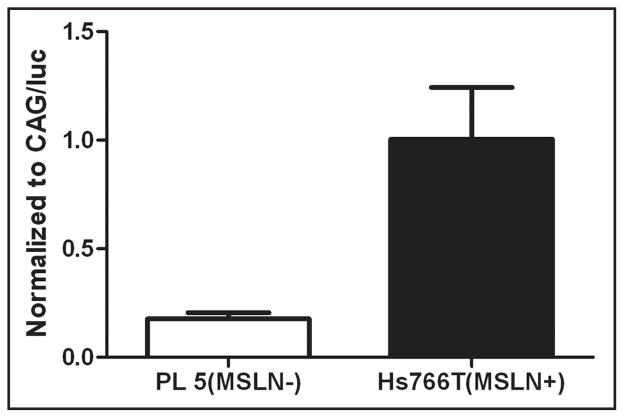
Using a MSLN promoter to regulate a luciferase reporter gene expression, we assessed MSLN promoter activity in MSLN+ and MSLN− pancreatic cancer cells. Luciferase activity was over 5-fold higher in the MSLN+ pancreatic cancer cell line than the MSLN− pancreatic cancer cell line (Fig. 2). This result suggests that differences in the expression of endogenous MSLN in these cell lines is not due to alterations in the MSLN promoter itself, but rather in differences in the transcriptional machinery of the cells.
Figure 2.
Specificity of MSLN promoter in MSLN+ and MSLN− pancreatic cancer cells based on luciferase expression. The MSLN promoter driving luciferase had a high amount of luciferase activity in the MSLN+ cancer cell line, Hs7766T, compared to the MSLN− line, PL5. To adjust for transfection efficiency between the cell lines, these data points were normalized to luciferase activity in cells transfected with CAG/Luc nanoparticles. Experiments were performed in triplicate (error bars represent SEM).
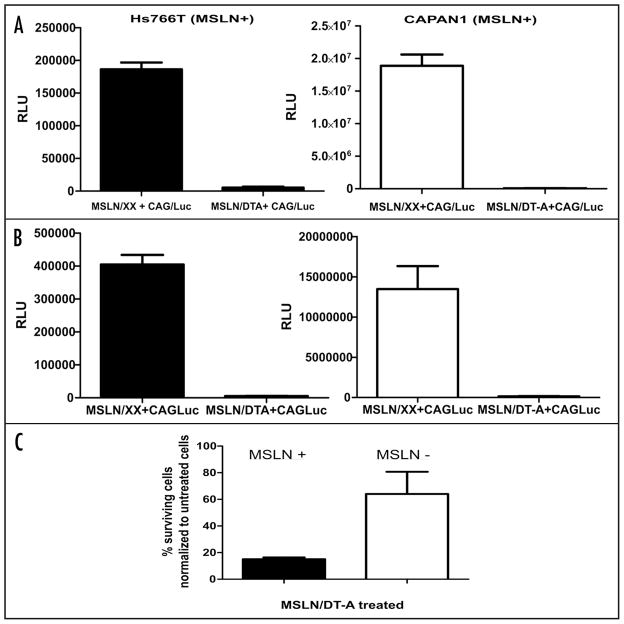
Nanoparticle delivery of DT-A DNA inhibits MSLN+ pancreatic cancer cells
Measuring luciferase enzyme activity in co-transfection experiments allows for the detection of a reduction of luciferase caused by the successful transfection of cells with DT-A encoding DNA, and thus monitoring for inhibition of protein synthesis.27 Transfection experiments were performed on two MSLN+ lines (Hs766T and CAPAN1), with the following combinations of nanoparticle-delivered DNA: (MSLN/XX + CAG/Luc), (MSLN/DT-A + CAG/Luc), and (pCI control DNA + CAG/Luc) (Fig. 3A and B). MSLN+ cell lines, Hs766T and CAPAN1, had a 97% and >99% reduction, respectively, in luciferase activity when comparing the (MSLN/XX + CAG/Luc) transfection with the (MSLN/DT-A + CAG/Luc) transfection just 24 hours after treatment (Fig. 3A). Similar results were achieved at 48 hours post-treatment in both cell lines (Fig. 3B). These experiments show that delivery of MSLN/DT-A DNA effectively inhibits protein synthesis in MSLN+ pancreatic cancer cells.
Figure 3.
Nanoparticle-DT-A DNA delivery to MSLN+ cells inhibits protein synthesis dramatically and affects overall cell survival. Luciferase activity measured after (A) 24 hour and (B) 48 hour post-delivering MSLN+ cell lines, Hs766T and CAPAN1, with MSLN/CAG-Luc DNA and MSLN/DT-A/CAG-luc DNA. Experiments were performed in triplicate (error bars represent SD, RLU = relative light units). (C) Cell survival assays of MSLN+ pancreatic cancer cells, Hs766T, and the MSLN− pancreatic cancer cell line, PL5. Total number of viable cells was enumerated manually 6 days post-delivery by trypan blue staining. Percent viability was determined by calculating total number of viable cells compared to untreated cultures. Experiments were performed in duplicate with two counts made for each well (Error bars represent SEM). We further determined that 34% of the total cells counted (viable plus dead) for the MSLN+ line on day 6 were dead, as assayed by trypan blue exclusion.
Previously, we assayed pancreatic and colon cancer cells for cell survival 5–7 days after treatment with a diverse class of clinically utilized chemotherapeutics and acquired relevant and informative in vitro data.29–31 Thus, in a set of similar experiments, we tested the effect of nanoparticle delivery of DT-A DNA on cell survival of MSLN+ pancreatic cancer cells in culture. Hs776T cells (MSLN+) and PL5 cells (MSLN−) were transfected with MSLN/DT-A DNA and both viable and dead cells were enumerated on day six and compared to untransfected cells. MSLN− cells transfected with MSLN/DT-A had a modest reduction in the total number of live cells as compared to cells that received no treatment, while the transfected MSLN+ cell line had nearly 85% reduction in viable cells, compared to the number of cells in the untreated group (Fig. 3C). Thus, the transfected Hs766T cells were more sensitive to the MSLN/DT-A treatment than the PL5 cells.
Discussion
MSLN overexpression is a hallmark of the majority of PDA cells as shown by us and numerous investigators (Fig. 1 and Table 1). In this current study, we report the in vitro exploitation of the naturally occurring transcriptional pathway that leads human pancreatic cancer cells to overexpress MSLN. The marked reduction of luceriferase activity (protein synthesis) in two MSLN+ pancreatic cancer cell lines (over 95% in both instances) following transfection with a MSLN/DT-A DNA construct establishes a novel paradigm for specifically targeting toxic molecules to pancreatic tumor cells. Since MSLN overexpression has been linked to various solid tumor types,6,16–18,32 we predict that these findings can be extended to other forms of cancer, as has already been accomplished in ovarian cancer cells by Sawicki and colleagues.
We demonstrate DT-A inhibition of protein synthesis and cell survival of MSLN+ pancreatic cancer cells. Theoretically, a single molecule of DT-A toxin is able to kill a host cell by inhibiting protein synthesis.20,33 Because the DT-B subunit is not included, the DT-A released from the dead cells is not able to enter surrounding cells.21 This strategy of targeting protein synthesis in pancreatic cancer cells is especially promising because the downstream functional effects of the common mutational events in pancreatic cancer, K-ras activation and inactivation of two tumor suppressors (p53 and Rb), have been previously shown to increase protein synthesis in other experimental models. These studies have shown that K-ras and the p53 and Rb pathways co-regulate the protein synthesis network. In pancreatic cancer, the oncogene K-ras is constitutively activated (i.e., turning on protein synthesis), while the tumor suppressor pathways, p53 and Rb (through p16 silencing), are inactivated and thus can not regulate protein synthesis.4,34–38 Loss and gain of function of these proteins would in theory affect the regulatory circuits of the pol III network that controls protein synthesis. Further, it has been shown that mTOR is a key regulator of protein synthesis and is overactive in pancreatic cancer cells.39 mTOR interacts with elongation factor 2 which is directly targeted by DT-A.33,40 Thus, targeting protein synthesis via pancreatic cancer cell-specific expression of DT-A DNA is a logical approach of targeting a possible collaborative functional outcome of the most frequent mutations (i.e., K-ras, p53 and p16) found in pancreatic tumors.4
In this study, we utilized a nanoparticulate delivery system that has already been translated in vivo for prostate cancer,22 and more recently, ovarian cancer. This modern mode of gene delivery is exciting because thorough investigation and refinement of these polymers have shown them to be safe, effective and biodegradable vehicles for suicide DNA delivery in vitro and in vivo.23 Using polymers to deliver suicide DNA has numerous advantages over more conventional viral-based vectors of gene therapy. Most importantly, this DNA delivery system is easy to produce, stable and highly efficient at delivering large amounts of DNA.21
One limitation to the therapeutic approach of using DT-A to kill cancer cells is the possibility of a ‘leaky’ promoter, and subsequent unwanted cell death in normal tissues. Further, in characterizing MSLN-reactive antibodies, Onda M. et al. detected no expression of MSLN in normal tissues including liver, lung, ovarian stroma, brain, breast, uterus (endometrium, myometrium), placenta and kidney tissues. Yet, MSLN expression was detected in the cancerous tissue, except for the peritoneal mesothelium (on uterus), pleural mesothelium.41 Thus, even with a cancer-specific promoter, normal cell death may occur when cellular mechanisms that control expression of toxic genes are not tightly controlled. Previous work has shown that the use of a dual regulatory system that combines transcription control with site-directed DNA recombination, successfully and specifically controls the expression of DT-A in cancer cells.27,42 We plan to use this strategy to assure pancreatic cancer specific targeting by utilizing cancer specific enhancer elements from the MSLN promoter14 and combining this with the Prostate Stem Cell Antigen (PSCA) promoter that would be specifically active in pancreatic cancer cells.43 If additional pre-clinical studies show errant DT-A expression, inclusion of this dual regulatory strategy into DNA constructs could be easily adopted.
Future studies will explore the efficacy of this therapeutic strategy in the K-ras/p53 pancreatic mouse model.44 We have recently determined that tumors in these mice express MSLN (our unpublished findings). Currently, we are also attempting to optimize the MSLN promoter by incorporating recent advances in our understanding of MSLN transcriptional regulation.14 Ultimately, testing pancreatic tumors post-operatively for MSLN expression (Fig. 1B–D) may stratify patients for potent targeted nanotherapy. Thus, nanotherapy may be a safe and effective way to manage pancreatic cancer patients in the neoadjuvant and adjuvant settings alone, or in combination with other targeted therapies.
Methods
Cell lines
Human pancreatic cancer cell lines, MiaPaCa-2, Capan-1, PL-5 and Hs766T were grown in cell culture. The cell lines were cultured in DMEM with high glucose (Invitrogen, Carlsbad, CA, USA) supplemented with 1% penicillin/streptomycin, 1% L-glutamine and 10% fetal bovine serum. All pancreatic cancer cell lines were donated by Dr. Scott Kern (Johns Hopkins University, Baltimore, MD, USA).
RT-PCR
Total RNA was extracted from cell lines using RNeasy Mini Kit (Qiagen, Valencia, CA). cDNA was synthesized from RNA using SuperScript II following the manufacturer’s protocols. PCR conditions and primer sequences are available on request.6 PCR products were resolved by electrophoresis using LB media with agarose gels and stained with ethidium bromide (Faster Better Media LLC, Hunt Valley, MD).24
DNA constructs
pMSLN/Luc, containing the firefly luciferase coding sequence under the control of the human MSLN promoter, was constructed as follows: The 1850 bp MSLN promoter was PCR amplified from human genomic DNA using the oligonucleotide sequence 5′-GCT ACT AGT GTT TTC ATC ATT GTC CGC AGC-3′ as the forward primer and 5′-ATA TAG ATC TGA GGG AGG CAC CGT GGG TCC AG-3′ as the reverse primer. These primers were analogous to those previously described,25 except that they contain nucleotides corresponding to restriction enzyme sites at their 5′-ends. PCR cycling conditions were: five minutes at 94°C, followed by 30 cycles of 30 s at 94°C, 30 s at 58°C and two minutes at 68°C, using Platinum Pfx Taq polymerase (Invitrogen, Carlsbad, CA). The PCR product was digested with SpeI and BglII and ligated to pGL4.10(luc2) plasmid (Promega, Madison, WI) that had been digested with NheI and BglII.
pMSLN/DT-A plasmid, containing the DT-A coding sequence under the control of the human MSLN promoter, was constructed as follows: The plasmid p22EDT1 containing the DT-A sequence (gift of A. Francis Stewart, EMBL Heidelburg) was digested with BglII and NotI. The 1334 bp fragment containing DT-A was ligated to the plasmid pMECA, which had been digested with BglII and NotI, to generate pMECA/DT-A. Following digestion of pMECA/DT-A with NheI and NotI, the released DT-A sequence was ligated to (NheI + NotI)-digested pIND plasmid (Clontech, Mountain View, CA) to form pIND/DT-A. To replace the luciferase coding sequence in a pMSLN/Fluc plasmid with DT-A, the luciferase sequence was excised by digestion with BglII and Xba I, and the remaining vector containing the MSLN promoter was ligated to a 1351 bp DT-A fragment resulting from digestion of pIND/DTA with BglII and XbaI. pCAG/Luc was constructed as previously described.21 pMSLN/XX is the MSLN promoter in front of an empty vector sequence.
Transfection procedure
Based on our RT-PCR analysis, the pancreatic cancer cell line Hs766T was chosen as the MSLN positive (MSLN+) cell line. The cell line PL-5 was found to have undetectable MSLN expression and was chosen as the MSLN negative (MSLN−) cell line for the majority of these studies. 1 x 105 cells were plated in a 24-well plate 24 hour prior to transfection. Nanoparticles were prepared as previously reported.21 In brief, polymer C32-117 and DNA were diluted with sodium acetate (pH 7.5). A 1:20 polymer:DNA solution was made and allowed to sit at room temperature for 10 minutes. Opti-MEM reduced serum medium (Invitrogen) was added to the polymer/DNA solution. Medium was removed from the cells and replaced with 1 ml of the nanoparticle solution. Cells were then incubated for three hours at 37°C. After three hours, the Opti-MEM medium was removed and replaced with standard growth media (DMEM, high glucose). All experiments were performed in triplicate.
Luciferase expression
Luciferase activity was measured from cellular extracts by a Moonlight 2010 luminometer (Analytical Luminesence Laboratory, San Diego, CA) at 48 and 72 hour post-transfection using a luciferase assay kit (Promega, Madison, WI). MSLN promoter specificity was verified by performing the following transfections on both MSLN+ and MSLN− cell lines: (1) no treatment, (2) CAG/Luc, and (3) MSLN/Luc. CAG is a robust, non-specific regulatory sequence consisting of the CMV enhancer and the chicken β-actin promoter.26
In order to determine the effectiveness of the delivery of DT-A to pancreatic cancer cells in vitro, we complexed CAG/Luc plasmid DNA and MSLN/DT-A DNA with polymer and transfected the complexes in two different experimental settings.
First, we tested the efficacy of DT-A expression in MSLN+ pancreatic cancer cells using a previously reported method to monitor inhibition of protein synthesis by directly measuring luciferase activity.27 1 × 105 MSLN+ cells were plated in 24-well plates 24-hours prior to transfection. According to the transfection protocol, cells were either incubated with (MSLN/XX + CAG/Luc) nanoparticles, MSLN/DT-A + CAG/Luc nanoparticles, CAG/Luc nanoparticles, or left untreated. All experiments were performed in triplicate. Luciferase activity was measured 24 and 48 hour post-transfection.
To assess the effect on cell survival, an equal number of Hs766T and PL5 cells were plated into 6-well plates on the day prior to transfection. According to the transfection protocol described above, cells were either incubated with MSLN/XX nanoparticles, MSLN/DT-A nanoparticles, or left untreated. On day six, viable and dead cells were counted using trypan blue staining. Experiments were performed in duplicate with two counts made for each well.
Tissue samples
The pancreatic tissue microarray containing 40 well characterized pancreatic ductal adenocarcinoma cases and 10 normal pancreatic tissues was immunohistochemically stained with antibody to MSLN. Formalin-fixed, paraffin-embedded blocks were processed for immunohistochemical analysis using heat antigen retrieval and avidin-biotin complex technique as previously described.28 MSLN antibody (sc-50427, Santa Cruz, Santa Cruz, CA) was incubated on the slides overnight using a 1:50-dilution followed by a biotin labeled mouse secondary antibody (Dako, Carpinteria, CA).
Acknowledgments
*Indicates surrounding normal pancreas tissue or other normal pancreas tissue we negative for MSLN expression. This work was funded by NIH grant R01 CA132091 (J.A.S.) and NIH grant EB00244 (D.G.A. and R.L.).
References
- 1.Bilimoria KY, Bentrem DJ, Ko CY, Stewart AK, Winchester DP, Talamonti MS. National failure to operate on early stage pancreatic cancer. Ann Surg. 2007;246:173–80. doi: 10.1097/SLA.0b013e3180691579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kleeff J, Michalski CW, Friess H, Buchler MW. Surgical treatment of pancreatic cancer: The role of adjuvant and multimodal therapies. Eur J Surg Oncol. 2007;33:817–23. doi: 10.1016/j.ejso.2007.01.022. [DOI] [PubMed] [Google Scholar]
- 3.Sener SF, Fremgen A, Menck HR, Winchester DP. Pancreatic cancer: a report of treatment and survival trends for 100,313 patients diagnosed from 1985–1995, using the National Cancer Database. J Am Coll Surg. 1999;189:1–7. doi: 10.1016/s1072-7515(99)00075-7. [DOI] [PubMed] [Google Scholar]
- 4.Hruban RH, Iacobuzio-Donahue C, Wilentz RE, Goggins M, Kern SE. Molecular pathology of pancreatic cancer. Cancer J. 2001;7:251–8. [PubMed] [Google Scholar]
- 5.Showalter SL, Showalter TN, Witkiewicz A, Havens R, Kennedy EP, Hucl T, Kern SE, Yeo CJ, Brody JR. Evaluating the drug-target relationship between thymidylate synthase expression and tumor response to 5-fluorouracil: Is it time to move forward? Cancer Biol Ther. 2008;7:986–94. doi: 10.4161/cbt.7.7.6181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Argani P, Iacobuzio-Donahue C, Ryu B, Rosty C, Goggins M, Wilentz RE, Murugesan SR, Leach SD, Jaffee E, Yeo CJ, Cameron JL, Kern SE, Hruban RH. Mesothelin is overexpressed in the vast majority of ductal adenocarcinomas of the pancreas: identification of a new pancreatic cancer marker by serial analysis of gene expression (SAGE) Clin Cancer Res. 2001;7:3862–8. [PubMed] [Google Scholar]
- 7.Watanabe H, Okada G, Ohtsubo K, Yamaguchi Y, Mouri H, Motoo Y, Wakabayashi T, Sawabu N. Expression of mesothelin mRNA in pure pancreatic juice from patients with pancreatic carcinoma, intraductal papillary mucinous neoplasm of the pancreas and chronic pancreatitis. Pancreas. 2005;30:349–54. doi: 10.1097/01.mpa.0000160281.56828.76. [DOI] [PubMed] [Google Scholar]
- 8.Maitra A, Adsay NV, Argani P, Iacobuzio-Donahue C, De Marzo A, Cameron JL, Yeo CJ, Hruban RH. Multicomponent analysis of the pancreatic adenocarcinoma progression model using a pancreatic intraepithelial neoplasia tissue microarray. Mod Pathol. 2003;16:902–12. doi: 10.1097/01.MP.0000086072.56290.FB. [DOI] [PubMed] [Google Scholar]
- 9.Iacobuzio-Donahue CA, Ashfaq R, Maitra A, Adsay NV, Shen-Ong GL, Berg K, Hollingsworth MA, Cameron JL, Yeo CJ, Kern SE, Goggins M, Hruban RH. Highly expressed genes in pancreatic ductal adenocarcinomas: a comprehensive characterization and comparison of the transcription profiles obtained from three major technologies. Cancer Res. 2003;63:8614–22. [PubMed] [Google Scholar]
- 10.Ryu B, Jones J, Blades NJ, Parmigiani G, Hollingsworth MA, Hruban RH, Kern SE. Relationships and differentially expressed genes among pancreatic cancers examined by large-scale serial analysis of gene expression. Cancer Res. 2002;62:819–26. [PubMed] [Google Scholar]
- 11.Sato N, Fukushima N, Maitra A, Iacobuzio-Donahue CA, van Heek NT, Cameron JL, Yeo CJ, Hruban RH, Goggins M. Gene expression profiling identifies genes associated with invasive intraductal papillary mucinous neoplasms of the pancreas. Am J Pathol. 2004;164:903–14. doi: 10.1016/S0002-9440(10)63178-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sato N, Maitra A, Fukushima N, van Heek NT, Matsubayashi H, Iacobuzio-Donahue CA, Rosty C, Goggins M. Frequent hypomethylation of multiple genes overexpressed in pancreatic ductal adenocarcinoma. Cancer Res. 2003;63:4158–66. [PubMed] [Google Scholar]
- 13.Hassan R, Laszik ZG, Lerner M, Raffeld M, Postier R, Brackett D. Mesothelin is overexpressed in pancreaticobiliary adenocarcinomas but not in normal pancreas and chronic pancreatitis. Am J Clin Pathol. 2005;124:838–45. [PubMed] [Google Scholar]
- 14.Hucl T, Brody JR, Gallmeier E, Iacobuzio-Donahue CA, Farrance IK, Kern SE. High cancer-specific expression of mesothelin (MSLN) is attributable to an upstream enhancer containing a transcription enhancer factor dependent MCAT motif. Cancer Res. 2007;67:9055–65. doi: 10.1158/0008-5472.CAN-07-0474. [DOI] [PubMed] [Google Scholar]
- 15.Li M, Bharadwaj U, Zhang R, Zhang S, Mu H, Fisher WE, Brunicardi FC, Chen C, Yao Q. Mesothelin is a malignant factor and therapeutic vaccine target for pancreatic cancer. Mol Cancer Ther. 2008;7:286–96. doi: 10.1158/1535-7163.MCT-07-0483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hassan R, Viner JL, Wang QC, Margulies I, Kreitman RJ, Pastan I. Anti-tumor activity of K1-LysPE38QQR, an immunotoxin targeting mesothelin, a cell-surface antigen overexpressed in ovarian cancer and malignant mesothelioma. J Immunother. 2000;23:473–9. doi: 10.1097/00002371-200007000-00011. [DOI] [PubMed] [Google Scholar]
- 17.Chang K, Pastan I. Molecular cloning and expression of a cDNA encoding a protein detected by the K1 antibody from an ovarian carcinoma (OVCAR-3) cell line. Int J Cancer. 1994;57:90–7. doi: 10.1002/ijc.2910570117. [DOI] [PubMed] [Google Scholar]
- 18.Scholler N, Fu N, Yang Y, Ye Z, Goodman GE, Hellstrom KE, Hellstrom I. Soluble member(s) of the mesothelin/megakaryocyte potentiating factor family are detectable in sera from patients with ovarian carcinoma. Proc Natl Acad Sci USA. 1999;96:11531–6. doi: 10.1073/pnas.96.20.11531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thomas AM, Santarsiero LM, Lutz ER, Armstrong TD, Chen YC, Huang LQ, Laheru DA, Goggins M, Hruban RH, Jaffee EM. Mesothelin-specific CD8(+) T cell responses provide evidence of in vivo cross-priming by antigen-presenting cells in vaccinated pancreatic cancer patients. J Exp Med. 2004;200:297–306. doi: 10.1084/jem.20031435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maxwell IH, Maxwell F, Glode LM. Regulated expression of a diphtheria toxin A-chain gene transfected into human cells: possible strategy for inducing cancer cell suicide. Cancer Res. 1986;46:4660–4. [PubMed] [Google Scholar]
- 21.Anderson DG, Peng W, Akinc A, Hossain N, Kohn A, Padera R, Langer R, Sawicki JA. A polymer library approach to suicide gene therapy for cancer. Proc Natl Acad Sci USA. 2004;101:16028–33. doi: 10.1073/pnas.0407218101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Peng W, Anderson DG, Bao Y, Padera RF, Jr, Langer R, Sawicki JA. Nanoparticulate delivery of suicide DNA to murine prostate and prostate tumors. Prostate. 2007;67:855–62. doi: 10.1002/pros.20576. [DOI] [PubMed] [Google Scholar]
- 23.Zugates GT, Peng W, Zumbuehl A, Jhunjhunwala S, Huang YH, Langer R, Sawicki JA, Anderson DG. Rapid optimization of gene delivery by parallel end-modification of poly(β-amino ester)s. Mol Ther. 2007;15:1306–12. doi: 10.1038/sj.mt.6300132. [DOI] [PubMed] [Google Scholar]
- 24.Brody JR, Calhoun ES, Gallmeier E, Creavalle TD, Kern SE. Ultra-fast high-resolution agarose electrophoresis of DNA and RNA using low-molarity conductive media. Biotechniques. 2004;37:598–602. doi: 10.2144/04374ST04. [DOI] [PubMed] [Google Scholar]
- 25.Breidenbach M, Rein DT, Everts M, Glasgow JN, Wang M, Passineau MJ, Alvarez RD, Korokhov N, Curiel DT. Mesothelin-mediated targeting of adenoviral vectors for ovarian cancer gene therapy. Gene Ther. 2005;12:187–93. doi: 10.1038/sj.gt.3302404. [DOI] [PubMed] [Google Scholar]
- 26.Ikawa M, Kominami K, Yoshimura Y, Tanaka K, Nishimune Y, Okabe M. Green fluorescent protein as a marker in transgenic mice. Dev Growth Diff. 1995;37:455–9. doi: 10.1046/j.1440-169X.1995.t01-2-00012.x. [DOI] [PubMed] [Google Scholar]
- 27.Peng W, Chen J, Huang YH, Sawicki JA. Tightly-regulated suicide gene expression kills PSA-expressing prostate tumor cells. Gene Ther. 2005;12:1573–80. doi: 10.1038/sj.gt.3302580. [DOI] [PubMed] [Google Scholar]
- 28.Brody JR, Witkiewicz A, Williams TK, Kadkol SS, Cozzitorto J, Durkan B, Pasternack GR, Yeo CJ. Reduction of pp32 expression in poorly differentiated pancreatic ductal adenocarcinomas and intraductal papillary mucinous neoplasms with moderate dysplasia. Mod Pathol. 2007;20:1238–44. doi: 10.1038/modpathol.3800974. [DOI] [PubMed] [Google Scholar]
- 29.Brody JR, Hucl T, Gallmeier E, Winter JM, Kern SE, Murphy KM. Genomic copy number changes affecting the thymidylate synthase (TYMS) gene in cancer: A model for patient classification to aid fluoropyrimidine therapy. Cancer Res. 2006;66:9369–73. doi: 10.1158/0008-5472.CAN-06-2165. [DOI] [PubMed] [Google Scholar]
- 30.Gallmeier E, Calhoun ES, Rago C, Brody JR, Cunningham SC, Hucl T, Gorospe M, Kohli M, Lengauer C, Kern SE. Targeted disruption of FANCC and FANCG in human cancer provides a preclinical model for specific therapeutic options. Gastroenterology. 2006;130:2145–54. doi: 10.1053/j.gastro.2006.03.016. [DOI] [PubMed] [Google Scholar]
- 31.van der Heijden MS, Brody JR, Gallmeier E, Cunningham SC, Dezentje DA, Shen D, Hruban RH, Kern SE. Functional defects in the fanconi anemia pathway in pancreatic cancer cells. Am J Pathol. 2004;165:651–7. doi: 10.1016/S0002-9440(10)63329-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hassan R, Bullock S, Premkumar A, Kreitman RJ, Kindler H, Willingham MC, Pastan I. Phase I study of SS1P, a recombinant anti-mesothelin immunotoxin given as a bolus IV infusion to patients with mesothelin-expressing mesothelioma, ovarian, and pancreatic cancers. Clin Cancer Res. 2007;13:5144–9. doi: 10.1158/1078-0432.CCR-07-0869. [DOI] [PubMed] [Google Scholar]
- 33.Collier RJ. Diphtheria toxin: mode of action and structure. Bacteriol Rev. 1975;39:54–85. doi: 10.1128/br.39.1.54-85.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.White RJ, Trouche D, Martin K, Jackson SP, Kouzarides T. Repression of RNA polymerase III transcription by the retinoblastoma protein. Nature. 1996;382:88–90. doi: 10.1038/382088a0. [DOI] [PubMed] [Google Scholar]
- 35.White RJ. Regulation of RNA polymerases I and III by the retinoblastoma protein: a mechanism for growth control? Trends Biochem Sci. 1997;22:77–80. doi: 10.1016/s0968-0004(96)10067-0. [DOI] [PubMed] [Google Scholar]
- 36.Stein T, Crighton D, Boyle JM, Varley JM, White RJ. RNA polymerase III transcription can be derepressed by oncogenes or mutations that compromise p53 function in tumours and Li-Fraumeni syndrome. Oncogene. 2002;21:2961–70. doi: 10.1038/sj.onc.1205372. [DOI] [PubMed] [Google Scholar]
- 37.Felton-Edkins ZA, Kenneth NS, Brown TR, Daly NL, Gomez-Roman N, Grandori C, Eisenman RN, White RJ. Direct regulation of RNA polymerase III transcription by RB, p53 and c-Myc. Cell Cycle. 2003;2:181–4. [PubMed] [Google Scholar]
- 38.Felton-Edkins ZA, Fairley JA, Graham EL, Johnston IM, White RJ, Scott PH. The mitogen-activated protein (MAP) kinase ERK induces tRNA synthesis by phosphorylating TFIIIB. Embo J. 2003;22:2422–32. doi: 10.1093/emboj/cdg240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pham NA, Schwock J, Iakovlev V, Pond G, Hedley DW, Tsao MS. Immunohistochemical analysis of changes in signaling pathway activation downstream of growth factor receptors in pancreatic duct cell carcinogenesis. BMC Cancer. 2008;8:43. doi: 10.1186/1471-2407-8-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Browne GJ, Proud CG. A novel mTOR-regulated phosphorylation site in elongation factor 2 kinase modulates the activity of the kinase and its binding to calmodulin. Mol Cell Biol. 2004;24:2986–97. doi: 10.1128/MCB.24.7.2986-2997.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Onda M, Willingham M, Nagata S, Bera TK, Beers R, Ho M, Hassan R, Kreitman RJ, Pastan I. New monoclonal antibodies to mesothelin useful for immunohistochemistry, fluorescence-activated cell sorting, Western blotting, and ELISA. Clin Cancer Res. 2005;11:5840–6. doi: 10.1158/1078-0432.CCR-05-0578. [DOI] [PubMed] [Google Scholar]
- 42.Peng W, Verbitsky A, Bao Y, Sawicki J. Regulated expression of diphtheria toxin in prostate cancer cells. Mol Ther. 2002;6:537–45. doi: 10.1006/mthe.2002.0694. [DOI] [PubMed] [Google Scholar]
- 43.Argani P, Rosty C, Reiter RE, Wilentz RE, Murugesan SR, Leach SD, Ryu B, Skinner HG, Goggins M, Jaffee EM, Yeo CJ, Cameron JL, Kern SE, Hruban RH. Discovery of new markers of cancer through serial analysis of gene expression: prostate stem cell antigen is overexpressed in pancreatic adenocarcinoma. Cancer Res. 2001;61:4320–4. [PubMed] [Google Scholar]
- 44.Hingorani SR, Wang L, Multani AS, Combs C, Deramaudt TB, Hruban RH, Rustgi AK, Chang S, Tuveson DA. Trp53R172H and KrasG12D cooperate to promote chromosomal instability and widely metastatic pancreatic ductal adenocarcinoma in mice. Cancer Cell. 2005;7:469–83. doi: 10.1016/j.ccr.2005.04.023. [DOI] [PubMed] [Google Scholar]