Abstract
The aim of this prospective study (20 months) was to assess HIV patients' use of Traditional, Complementary and Alternative Medicine (TCAM) and its effect on ARV adherence at three public hospitals in KwaZulu-Natal, South Africa. Seven hundred and thirty-five (29.8% male and 70.2% female) patients who consecutively attended three HIV clinics completed assessments prior to ARV initiation, 519 after 6 months, 557 after 12 and 499 after 20 months on antiretroviral therapy (ART). Results indicate that following initiation of ARV therapy the use of herbal therapies for HIV declined significantly from 36.6% prior to ARV therapy to 8.0% after 6 months, 4.1% after 12 months and 0.6% after 20 months on ARVs. Faith healing methods (including spiritual practices and prayer) declined from 35.8% to 22.1%, 20.8% and 15.5%, respectively. In contrast, the use of micronutrients (vitamins, etc.) significantly increased from 42.6% to 78.2%. The major herbal remedies that were used prior to ART were unnamed traditional medicine, followed by imbiza (Scilla natalensis planch), canova (immune booster), izifozonke (essential vitamins mixed with herbs), African potato (Hypoxis hemerocallidea), stametta (aloe mixed with vitamins and herbs) and ingwe (tonic). Herbal remedies were mainly used for pain relief, as immune booster and for stopping diarrhea. As herbal treatment for HIV was associated with reduced ARV adherence, patient's use of TCAM should be considered in ARV adherence management.
Keywords: Traditional, complementary, alternative medicine, antiretroviral treatment adherence, prospective study, HIV patients, KwaZulu-Natal, South Africa
Introduction
Traditional, complementary and alternative medicine (TCAM) broadly comprises herbal remedies, spiritual practices and prayer, traditional Chinese medicines, acupuncture, acupressure, chiropractic care, massage therapy, meditation, visualization, therapeutic touch and micronutrients (vitamins, minerals, and multivitamins). TCAM has been demonstrated to be widely used by people living with HIV (PLHIV), both on and off ARV therapy, as primary treatment for HIV/AIDS and for HIV-related problems (e.g., Babb et al., 2007; Josephs et al., 2007; Langlois-Klassen et al., 2007; Ma et al., 2008; Malangu, 2007; Mills et al., 2005; Peltzer et al., 2008, 2010; Reid et al., 2008). Numerous medicinal plants used by traditional medicine practitioners for the treatment of HIV/AIDS and related conditions in sub-Saharan Africa have been identified (Klos et al., 2009; Lamorde et al., 2010; Theo et al., 2009) including Hypoxis hemerocallidea (African potato) and Sutherlandia (Mills et al., 2005a) and micronutrient interventions (Friis, 2006) which could be used as potential therapy for HIV.
There is data to suggest that TCAM impacts on ARV adherence, although the findings are variable and findings are inconsistent across studies (Littlewood and Vanable, 2008; Owen-Smith et al., 2007; Peltzer et al., 2010). There seem to be no longer-term prospective studies on the use of TCAM and its impact on antiretroviral treatment (ART) adherence in Africa. Therefore, the aim of this longer-term prospective study was to assess HIV patients' use of TCAM and its effect on ARV adherence over a 20 months period at three public hospitals in KwaZulu-Natal, South Africa. Previous studies (with the same sample) had assessed only global categories of traditional, complementary and alternative medicine for HIV at baseline and six months follow-up (Peltzer et al., 2008; Peltzer et al., 2010), while this report describes the details of each traditional, complementary and alternative medicine used and reasons of its use over 4 time points (including 12 and 20 months follow-up) and ART adherence at 12 and 20 months follow-up.
Methods
This was a prospective study of all adult treatment-naïve patients (N = 735) recruited from the three public hospitals in Uthukela health district in KwaZulu-Natal, South Africa from October 2007 to February 2008. All ARV-naïve patients who were about to start ARVs (18 years and above) and who consecutively attended the HIV clinics during the recruitment period were eligible for this study. Details of the setting, sampling procedure and recruitment have been described elsewhere (Peltzer et al., 2008). Patients were interviewed at 6, 12 and 20 months clinic visits post initiation of ARV follow-up. Patients who failed to attend the planned follow-up were contacted by telephone and up to two home visits before being considered lost to follow-up. Ethics approval was obtained from the Human Sciences Research Council (HSRC) ethics committee. Permission to conduct the study was obtained from the Provincial Department of Health in KwaZulu-Natal. Informed consent was obtained from each participant.
Measures
Patients were interviewed using an anonymous questionnaire administered by the research team. Information was collected on socio-demographic characteristics, clinical history and health-related characteristics, TCAM use and health beliefs. Following initiation of ARVs, information on side effects, and changing or interrupting ART was also obtained. The use of TCAM was assessed with questions on 8 different TCAM methods over the past six months, the duration of usage, costs and the awareness of the health care provider of using TCAM. Further, participants were asked for what reasons that had used each specific TCAM method. The response options included (a) To treat adverse effects from ARV treatment (e.g. nausea/vomiting), (b) Relaxation, (c) Pain relief, (d) Stress relief, (e) Immune supplementation, (f) Antioxidant, (g) Improve eating, (h) improve overall well-being, (i) Anxiety, (j) Depression, (k) Fatigue, (l) Neuropathy, (m) Diarrhoea, and (i) Other (specify). Clinical data relating to date of HIV diagnosis, HIV acquisition and transmission risk factors, current CD4 cell count, viral load (Chiron 3.0 bDNA), opportunistic infections, HIV and non-HIV medications was obtained from the medical chart.
Adherence assessment
The 30-day visual analog scale (VAS) provided an overall adherence assessment for a longer time interval. The VAS is a valid method of assessing medication adherence (Kalichman et al., 2009) and has been validated in resource-limited settings (e.g. Maneesriwongul et al., 2006). Adherence was calculated as the % of doses taken over those prescribed. Adherence levels assessed from the VAS are defined as follows: full adherence = 100%, partial adherence >/= 95% and < 100%, and non-adherence as < 95% of prescribed doses taken since the last refill.
Data analysis
Scientific names of herbal and other indigenous remedies were as far as possible identified using “HEALTHLIT”, a database including a Dictionary of South African Traditional Medicinal Plants and Zulu Medicinal Plants (Hutchings et al., 1996). Data were analyzed using Statistical Package for the Social Sciences (SPSS) for Windows software application programme version 17.0. Frequencies, means, standard deviations, median and interquartile ranges were calculated to describe the sample. Bivariate analyses using logistic regression were conducted to examine the relationships between overall TCAM use, herbal remedy use and socio-demographic variables, health characteristics, ART adherence and further health-seeking behaviour variables. Multivariate logistic regression included in the model all variables statistically significant at the 5% level in bivariate analyses. No significant interactions were found between socio-demographic variables health-seeking behaviour and health characteristics. In multivariate regressions for ART adherence, adjustment was made for site of care, to account for variations in practice patterns and demographic differences across sites.
Results
A total of 735 patients (217 men and 518 women) completed a baseline questionnaire at Time 1 prior to initiating ART. Follow-up questionnaires were completed at 6 months follow-up by 519 patients within this cohort (139 men and 370 women) who had now been on ART for six months, at 12 months later by 557 patients within this cohort (157 men and 396 women), and 20 months later by 499 patients (126 men and 333 women); 122 (16.9%) participants were lost-to-follow-up (including transfers), and 83 (11.5%) were known to have died, of whom, 75 had already died at six months follow-up, 72 transferred care elsewhere, 14 refused participation, 12 were not initiated on ART and 50 (6.9%) could not be traced. At time 4, HIV medications for 380 (76.3%) patients included Lamivudine (3TC), Stavudine (d4T) + Efavirenz and for 118 (23.7%) Lamivudine (3TC), Stavudine (d4T) + Nevirapine.
Sample characteristics
The mean age of the participants at baseline assessment was 35.9 years, std deviation = 9.7 years, the educational level of the majority (81.0%) was less than Grade 12. Almost three quarters (71.8%) were never married, 61.2% were unemployed and 20.2% employed, more than half (52.3%) had a child care grant, 29.9% had a formal salary and 7.9% had no income. Almost two-thirds (63.3%) resided in a rural area, most (73.5%) had been recently (within the past year) diagnosed as being HIV positive and almost all (96.1%) had disclosed their HIV status to someone. While baseline characteristics were similar between men and women, women were more likely to be younger and receiving social (including child care) grants, and men were more likely to be married or cohabitating, employed and having a formal salary. The median CD4 count at 20 months follow-up was 446 cells/cu.mm compared to 261 cells/cu.mm at 12 months follow-up, 130 cells/cu.mm at 6 months follow-up and compared to 119 cells/cu.mm prior to ARV initiation (see Table 1).
Table 1.
Sample characteristics of patients at baseline assessment
| Variable | N=735 | % |
| Sex | ||
| Male | 217 | 29.5 |
| Female | 518 | 70.5 |
| Age, range 18–67 | 35.9 | 9.7 |
| Age, less than 36 | 417 | 56.7 |
| Education | ||
| Grade 7 or less | 279 | 37.8 |
| Grade 8–11 | 314 | 42.8 |
| Grade 12 or more | 139 | 18.9 |
| Missing | 3 | 0.4 |
| Religion | ||
| Traditional African religion or none | 187 | 25.5 |
| Main stream Christian | 154 | 20.9 |
| Charismatic | 271 | 36.9 |
| Other | 123 | 16.7 |
| Residence | ||
| Rural (village) | 338 | 46.0 |
| Rural (farm) | 125 | 17.0 |
| Urban (informal settlements) | 41 | 5.6 |
| Urban (formal settlements) | 227 | 30.9 |
| Missing | 4 | 0.5 |
| Employment status | ||
| Housewife/houseman | 99 | 13.5 |
| Unemployed | 448 | 60.9 |
| Employed | 148 | 20.2 |
| Pensioner/disabled/student | 25 | 3.4 |
| Other | 12 | 1.6 |
| Missing | 3 | 0.4 |
| Income | ||
| Formal salary | 29.3 | |
| Family member contributions | 215 | 18.1 |
| Social grants | 133 | 35.9 |
| 264 | ||
| No income | 57 | |
| Other | 51 | 7.8 |
| Missing | 15 | 6.9 |
| 2.0 | ||
| Time since HIV diagnosis | ||
| ≤1 year (2007/8) | 540 | 73.5 |
| 1–2 years (2006) | 73 | 9.9 |
| >2 years (2005–1995) | 122 | 16.6 |
| CD4 count (cells/uL)= T4: Median=446 (IQR=305–618), T3: Median=261 (IQR=176–387), T2=130 (IQR=72–185) (at baseline: 119; IQR=59–163) | ||
| 1–99 | 106 | 19.7 |
| 100–349 | 345 | 64.2 |
| ≥350 | 86 | 16.0 |
TCAM use
Following initiation of ARV therapy the use of herbal therapies for HIV declined significantly from 36.6% prior to ARV therapy to 8.0% after 6 months, 4.1% after 12 months and 0.6% after 20 months on ARVs. Faith healing methods (including spiritual practices and prayer) declined from 35.8% to 22.1%, 20.8% and 15.5% respectively, and physical/body-mind therapy (exercise and massage) declined from 5.0% to 1.9%, 0.5% and 0.6%. In contrast, the use of micronutrients (vitamins, etc.) significantly increased from 42.6% to 93.8% at 12 months but then decreased to 78.2% at 20 months. At baseline only 10.2% of patients reported that their health care provider was aware of their herbal remedy use. This figure declined to 4.7% after 6 months, 4.2% after 12 months and 0% after 20 months on ARVs. Patients were more willing to disclose faith healing methods than herbal remedies to their health care provider, with the disclosure rate increasing from 16.1% at baseline to 35.1% at 20 months follow-up (see Table 2).
Table 2.
Frequency distribution of the use, costs, awareness of the care provider of different TCAM (Traditional, complementary and alternative medicine) for HIV in the past six months (prior to ART=Time 1(T1), N=735; follow-up one (T2), N=525); follow-up two (T3) (n=556); and follow-up three (T4) (n=499)
| Use duration in weeks |
Cost in Rand/month |
Health care provider aware of use |
||||
| N | % | M (SD) | M (SD) | n (%) | ||
|
1. Herbal therapies (e.g., Ginseng, Echinacea or St. John's Wort, Hypoxis plant (African potato), cannabis) |
T1 T2 T3 T4 |
269 43 23 03 |
36.6 8.0 4.1 0.6 |
10.0 (8.1) 4.0 (1.5) 5.6 (2.0) 5.0 (2.8) |
129 (166) 250 (330) 77 (80) 65 (7.1) |
21 (10.2) 02 (4.7) 01 (4.2) 03 (0.0) |
|
2. Faith healing including spiritual practices and prayer |
T1 T2 T3 T4 |
263 116 116 77 |
35.8 22.1 20.8 15.5 |
11.9 (7.0) 19.8 (18.6) 8.4 (12.1) 4.1 (5.3) |
0.4 (1.0) 0.2 (1.7) 1.3 (5.1) 1.0 (2.4) |
42 (16.1) 58 (50.4) 37 (31.6) 27 (35.1) |
|
3. Physical/body-mind therapy (e.g. exercise, massage) |
T1 T2 T3 T4 |
37 10 03 03 |
5.0 1.9 0.5 0.6 |
13.5 (14.8) 8.9 (6.7) 5.5 (2.7) 3.6 (2.8) |
9 (11) 0.8 (2.6) 0.0 0.0 |
02 (0.5) 06 (60.0) 02 (66.6) 01 (33.3) |
|
4. Micronutrients (vitamins, minerals & multivitamin) |
T1 T2 T3 T4 |
312 457 525 388 |
42.6 87.4 93.8 78.2 |
12.5 (9.5) 18.3 (12.4) 22.0 (13.6) 16.2 (9.0) |
6 (53) 8 (20) 12.2 (20.2) 36.4 (32.0) |
173 (66.3) 449 (98.3) 508 (95.3) 388 (100) |
| 5. Over-the-counter drugs | T1 T2 T3 T4 |
15 48 14 04 |
2.1 6.5 2.5 0.8 |
14.2 (14.1) 4.8 (2.1) 7.0 (5.6) 1.7 (1.7) |
159 (202) 23 (22.3) 45 (20.3) 15 (12.3) |
04 (22.2) 28 (58.3) 13 (72.2) 04 (100) |
aThe use of micronutrients was excluded from TCAM since mostly vitamins were provided by the health facility
The major herbal remedies that were used prior to ART were unnamed traditional medicine (57), followed by imbiza (43), canova (31), izifozonke (21), African potato (15), stametta (14) and ingwe (10). The use of African potato (9), canova (8) and izifozonke (7) proportionally continued to time 2 (6 months on ART), while the use of stametta (1) and ingwe (2) drastically reduced. Herbal remedy use at time 3 (12 months on ART) was mainly stametta (5), canova (3) and Tre-en-en (4), while African potato, imbiza, ingwe, izifozonke were no longer used, and at 20 months follow-up the use of herbal remedies had virtually stopped. Participants reported the use of herbal remedies for a number of purposes, mainly pain relief, immune booster and stopping diarrhea. Over time it appears the use of herbal remedies for pain relief reduced (African potato, ingwe, izifozonke). Herbal remedies were often obtained over-the-counter (e.g. canova, stametta, ingwe), some from both the traditional healer and over-the-counter (e.g. African potato, izifonzonke) and from the traditional health practitioner only (e.g. unnamed traditional medicine) (see Table 3).
Table 3.
The frequency of the use, source and purpose of each TCAM over time (baseline, 6, 12, and 20 months on ART)
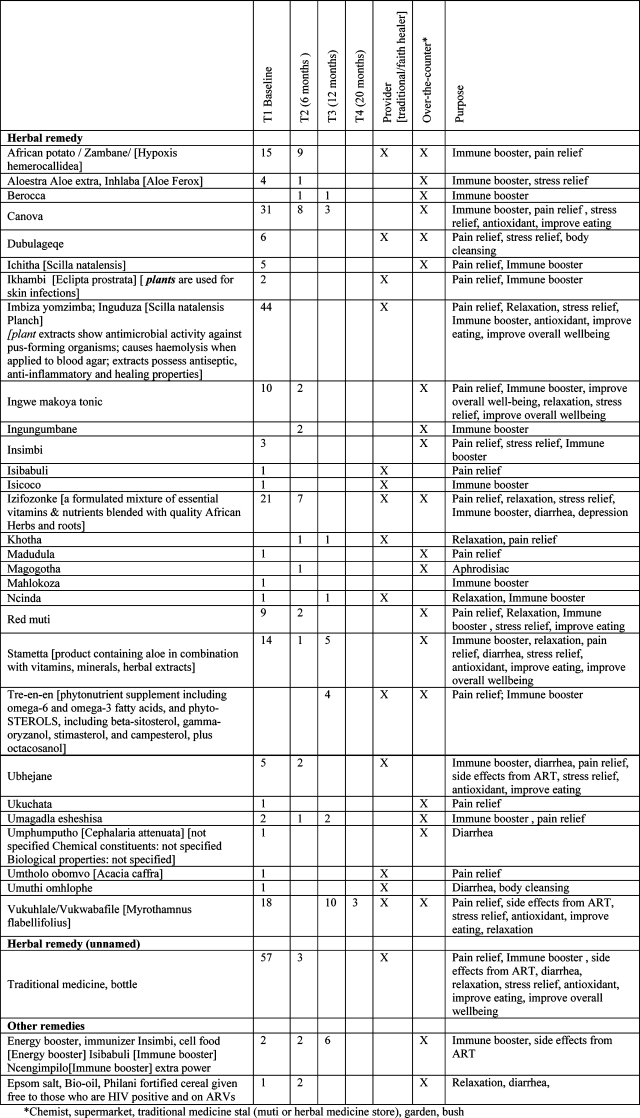 |
Overall, herbal therapies were believed to be used at 6.1% at time 1 to treat adverse effects of HIV, and at time 2 and 3 (4.3% and 3.3% respectively) for treating adverse effects from ARV treatment. Over time herbal remedies were used at time 1 for pain relief (27.9%), to improve eating (14.7%), improve overall well-being (13.2%), as an antioxidant (9.9%) and for stress relief (17.3%). At time 2 and 3 herbal remedies were used for immune supplementation (65% and 70%). Faith healing was primarily used to improve overall well-being and stress relief, similarly over time. Physical/body-mind therapy was mainly used for stress relief and relaxation, and micronutrients for immune supplementation and improving the overall well being. Finally, over-the-counter drugs were mainly used for pain relief. The belief that TCAM can be solely used to treat HIV was shared by 6.1% prior to ART, 1.5% at 6 months and 4.8% at 12 months on ART (see Table 4).
Table 4.
Frequency distribution of the reasons for different TCAM use (multiple responses possible) over time
| T1 (n=735) T2 (n=525) T3 (n=556) T4 (n=499) |
1. Herbal therapies |
2. Faith healing including spiritual practices and prayer |
3. Physical/bodymind therapy (e.g. exercise, massage) |
4. Micronutrients (vitamins, minerals & multivitamin) |
5. Over-the-counter drugs |
| To treat adverse effects from HIV or ARV treatment(e.g. nausea/vomiting) |
T1=45 (6.1) T2=2 (4.3) T3=1 (3.3) T4=0 |
T1=15 (2.0) T2=5 (2.1) T3=24 (12.1) T4=1 (0.8) |
T1=3 (0.4) T2=1 (8.3) T3=11 (35.5) T4=0 |
T1=3(4.1) T2=9 (1.9) T3=59 (9.1) T4=11 (3.0) |
T1=# T2=3 (5.4) T3=5 (22.7) T4=0 |
| Relaxation | T1=59 (8.0) T2=5 (10.9) T3=4 (13.3) T4=0 |
T1=68 (9.3) T2=10 (4.2) T3=6 (3.0) T4=2 (1.6) |
T1=13 (1.8) T2=3 (25.0) T3=3 (9.7) T4=3 (0.9) |
T1=92 (12.5) T2=1 (0.2) T3=2 (0.3) T4=1 (0.3) |
T1=# T2=1 (1.8) T3=0 T4=0 |
| Pain relief | T1=205 (27.9) T2=5 (10.9) T3=3 (10.0) T4=0 |
T1=134 (18.2) T2=9 (3.8) T3=2 (1.0) T4=1 (0.8) |
T1=14 (1.8) T2=0 T3=1 (3.2) T4=0 |
T1=259 (35.2) T2=9 (1.9) T3=5 (0.8) T4=0 |
T1=# T2=42 (75.0) T3=12 (54.5) T4=16 (84.2) |
| Stress relief | T1=127 (17.3) T2=2 (4.3) T3=0 T4=0 |
T1=160 (21.8) T2=53 (22.3) T3=23 (18.7) T4=0 |
T1=15 (2.0) T2=5 (41.7) T3=5 (16.1) T4=1 (0.8) |
T1=152 (20.7) T2=3 (0.6) T3=2 (0.3) T4=0 |
T1=# T2=1 (1.8) T3=2 (9.1) T4=0 |
| Immune supplementation |
T1=132 (18.0) T2=30 (65.2) T3=21 (70.0) T4=3 (100) |
T1=101 (13.7) T2=9 (3.8) T3=19 (9.5) T4=1 (0.8) |
T1=13 (1.8) T2=1 (8.3) T3=9 (29.0) T4=4 |
T1=182 (24.8) T2=284 (59.3) T3=244 (37.7) T4=126 (34.6) |
T1=# T2=4 (7.1) T3=1 (4.5) T4=0 |
| Antioxidant | T1=73 (9.9) T2=0 T3=0 T4=0 |
T1=84 (11.4) T2=1 (0.4) T3=0 T4=0 |
T1=4 (0.5) T2=0 T3=1 T4=0 |
T1=111 (15.1) T2=2 (0.4) T3=0 T4=0 |
T1=# T2=0 T3=0 T4=0 |
| Improve eating | T1=108 (14.7) T2=0 T3=0 T4=0 |
T1=92 (12.5) T2=2 (0.8) T3=2 (1.0) T4=0 |
T1=8 (1.1) T2=0 T3=0 T4=0 |
T1=112 (15.2) T2=71 (14.8) T3=121 (18.7) T4=81 (22.3) |
T1=# T2=0 T3=2 (9.1) T4=0 |
| Improve overall well being |
T1=97 (13.2) T2=1 (2.2) T3=1 (3.3) T4=0 |
T1=179 (24.4) T2=120 (50.4) T3=105 (52.8) T4=78 (63.4) |
T1=21 (2.9) T2=2 (16.7) T3=1 (3.2) T4=0 |
T1=154 (21.0) T2=100 (20.9) T3=208 (32.1) T4=145 (39.8) |
T1=# T2=0 T3=0 T3=10 |
| Anxiety | T1=0 T2=0 T3=0 T4=0 |
T1=0 T2=0 T3=1 (0.5) T4=0 |
T1=0 T2=0 T3=1 (3.2) T4=0 |
T1=0 T2=0 T3=1 (0.2) T4=0 |
T1=# T2=5 (8.9) T3=0 T4=0 |
| Depression | T1=0 T2=1 (2.2) T3=0 T4=0 |
T1=0 T2=29 (12.2) T3=14 (7.0) T4=5 (4.1) |
T1=0 T2=0 T3=0 T4=0 |
T1=0 T2=0 T3=4 (0.6) T4=0 |
T1=# T2=0 T3=0 T4=0 |
#Reasons for over-the-counter drug use was not assessed at baseline
ART Adherence
At 12 and 20 months using the VAS 89.6% and 91.6%, respectively, reported ART adherence at ≥95%. Bivariate analyses for ART adherence at time 3 (12 months follow-up) found that belonging to traditional African or no religion, urban residence, being a housemaker, having a formal salary, higher CD4 count, the non-use of herbs and faith healing were associated with ART adherence at 12 months, while in multivariate analysis urban residence (OR: 3.7, 1.6–8.8), being employed (OR: 6.5, 1.2–34.4) or unemployed (OR: 5.9, 1.4–25.7), belonging to a main stream Christian church (OR: 2.3, 1.0–5.3) and non-use of herbs (OR: 0.2, 0.1–0.5) were retained. Further, bivariate analyses for ART adherence at time 4 (20 months follow-up) found that belonging to a main stream Christian church, being unemployed, no formal salary income, non-use of herbs, faith healing and higher CD4 count were associated with ART adherence at 20 months, while in multivariate analysis higher CD4 counts (OR: 4.7, 1.2–19.0) and no income (OR: 4.8, 1.2–19.5) were retained (see Table 5).
Table 5.
Association between TCAM use, socio-demographic variables, CD4 cell count and antiretroviral adherence (>95%) at time 3 (12 months follow-up) and time 4 (20 months follow-up)
| Adherence at time 3 | Adherence at time 4 | |||
| VAS Cr OR |
VAS AORa |
VAS Cr OR |
VAS AORb |
|
| Men Women |
1.00 0.94 (0.51–1.70) |
--- | 1.00 0.67 (0.33–1.36) |
--- |
| Age | 1.02 (0.99–1.05) | --- | 0.99 (0.96–1.02) | --- |
| Married/cohabitating Never married |
1.00 0.98 (0.53–1.80) |
--- | 1.00 1.08 (0.54–2.13 ) |
--- |
|
Education Grade 7 or less Grade 8–11 Grade 12 or more |
1.00 1.13 (0.62–2.07) 0.96 (0.45–2.06) |
--- | 1.00 1.51 (0.74–3.10) 1.15 (0.46–2.84) |
--- --- |
|
Religion African or none Main stream Christian Charismatic |
1.00 0.38 (0.18–0.79)** 0.49 (0.25–0.95)* |
1.00 2.32 (1.01–5.33)* 1.99 (0.96–4.85) |
1.00 2.24 (1.00–5.04)* 1.34 (0.63–2.87) |
1.00 2.32 (0.96–5.63) 1.58 (0.69–3.60) |
|
Residence Rural Urban |
1.00 4.90 (2.18–11.02)*** |
1.00 3.71 (1.56–8.83)** |
1.00 1.50 (1.75–3.02) |
--- |
|
Employment status Housewife/houseman Unemployed Employed |
1.00 0.12 (0.03–0.52)** 0.20 (0.04–0.92)* |
1.00 5.88 (1.35–25.69)* 6.52 (1.23–34.40)* |
1.00 11.26 (1.27–14.07)* 1.51 (0.35–6.48) |
1.97 (0.56–6.98) 1.95 (0.38–9.93) |
|
Income Formal salary Family contributions Social grants No income |
1.00 0.89 (0.35–2.29) 0.45 (0.23–0.89)* 0.23 (0.09–0.58)** |
1.00 1.21 (0.39–3.74) 1.58 (0.65–3.89) 2.48 (0.74–8.26) |
1.00 3.38 (1.16–9.80)* 3.52 (1.37–9.03)** 8.57 (2.77–26.56)*** |
2.86 (0.83–9.92) 3.07 (0.98–9.54) 4.78 (1.17–19.54)* |
| Herbal treatment | 0.08 (0.04–0.15)*** | 0.18 (0.07–0.50)*** | 0.04 (0.00–0.50)* | 0.11 (0.01–1.55) |
| Faith healing | 5.37 (1.65–17.48)** | 2.88 (0.84–9.81) | 12.78 (1.74–93.86)* | 6.78 (0.98–51.89) |
|
CD4 100 ref 101–350 >350 |
1.00 1.86 (1.02–3.41)* 5.97 (1.70–20.93)** |
1.00 1.48 (0.73–2.98) 3.31 (0.88–12.43) |
1.00 2.29 (0.66–7.90) 6.72 (1.94–23.31)** |
1.00 2.19 (0.55–8.82) 4.74 (1.18–18.95)* |
Significant at *P <.05, **P<.01, ***P<.001
Hosmer and Lemeshow Chi-square 10.97, p.20; Cox & Snell R2 .13; Nagelkerke R2 .27
Hosmer and Lemeshow Chi-square 5.39, p.72; Cox & Snell R2 .08; Nagelkerke R2 .19
Discussion
In this prospective study following initiation of ARV therapy the use of herbal therapies and faith healing methods (including spiritual practices and prayer) for HIV declined significantly from prior to ARV therapy to after 6, 12 and 20 months on ARVs, and the use of micronutrients (vitamins, etc.) significantly increased over time. Both herbal and faith healing declined with longer time on ART, which may indicate the effectiveness of ART. The use of micronutrients increased mainly because they were provided in the medical setting.
The study found that major herbal remedies that were used for HIV were unnamed traditional medicine, imbiza, canova, izifozonke, African potato, stametta, ingwe, vukuhlale and aloe. Similarly, Babb et al. (2007) found in a workplace ART clinic in South Africa of 14 patients receiving ART and concomitantly using traditional medicines, it was reported that a total of 22 products, most frequently African potato and Aloe vera were used. In this study patients had used the African potato but later stopped it. Sutherlandia was not used although it is commonly used in other populations. Mills et al. (2005b) examined the effects of two African herbal medicines recommended for HIV/AIDS patients on antiretroviral metabolism. Extracts from Hypoxis and Sutherlandia showed significant effects on cytochrome P450 3A4 metabolism and activated the pregnane X receptor approximately twofold. P-glycoprotein expression was inhibited, with Hypoxis showing 42–51% and Sutherlandia showing 19–31% of activity compared with verapamil. Initiating policies to provide herbal medicines with antiretroviral agents may put patients at risk of treatment failure, viral resistance or drug toxicity. Yet Brown et al. (2008) found that Hypoxis hemerocallidea and l-canavanine interact with the efflux of nevirapine across intestinal epithelial cells and therefore can potentially increase the bioavailability of this antiretroviral drug when taken concomitantly. Thus far, biomedical evidence has revealed that ‘African potato’ extracts possess anti-inflammatory, antineoplastic, antioxidant, antidiabetic and antiinfective properties in vivo and in vitro. But more laboratory and clinical studies are required to clarify these observations, and to isolate, purify and characterize the active chemical constituents responsible for the herb's pharmaco-therapeutic effects (Owira and Ojewole, 2008). Canova was widely used in this study, it is a homeopathic product, produced according to the Hahnemannian homeopathic method, and seems to stabilize platelet morphology in HIV/AIDS (Smit et al., 2009). There is a need to provide information of the safety and efficacy of different TCAM methods.
The study found that most health care providers were not aware of the use of herbal treatment of their patients, as reported by the patients in this study, this even decreased over time. Reasons for this could be the explicit training patients receive on being discouraged to use herbal treatments and because of social desirability. On the other hand health care providers became more aware over time on the use of faith healing and over-the-counter drugs of their patients. Interventions targeted towards both health care provider and patients many enhance communication of TCAM use, avoid potential adverse events and drug interactions, and enhance ART adherence (Liu et al., 2009).
Limitations
One limitation of this study is the self-report data for both TCAM use and HAART adherence. In addition, only patients and not health workers were interviewed, which could have provided their perspective on TCAM use among their patients. Viral load data were only available for a few participants, and was therefore excluded from the analysis. Finally, the findings are derived from a sample of men and women residing in one district in one province in South Africa. Thus caution is urged in generalizing the findings to other districts and provinces in the country.
Conclusion
High use of TCAM prior to ART seems to continuously decline over 20 months when on ART. Herbal treatment for HIV was associated with non-adherence to ART and should be considered in ART adherence management.
Acknowledgement
We thank the TIBOTEC REACH initiative for funding this study.
References
- 1.Babb DA, Pemba L, Seatlanyane P, Charalambous S, Churchyard GJ, Grant AD. Use of traditional medicine by HIV-infected individuals in South Africa in the era of antiretroviral therapy. Psychol Health Med. 2007;12(3):314–320. doi: 10.1080/13548500600621511. [DOI] [PubMed] [Google Scholar]
- 2.Brown L, Heyneke O, Brown D, van Wyk JP, Hamman JH. Impact of traditional medicinal plant extracts on antiretroviral drug absorption. J Ethnopharmacol. 2008;119(3):588–592. doi: 10.1016/j.jep.2008.06.028. [DOI] [PubMed] [Google Scholar]
- 3.Friis H. Micronutrient interventions and HIV infection: a review of current evidence. Trop Med Int Health. 2006;11(12):1849–1857. doi: 10.1111/j.1365-3156.2006.01740.x. [DOI] [PubMed] [Google Scholar]
- 4.Hutchings A. Zulu medicinal plants: an inventory. Pietermaritzburg: University of Natal Press; 1996. [Google Scholar]
- 5.Kalichman SC, Amaral CM, Swetzes C, Jones M, Macy R, Kalichman MO, Cherry C. A simple single-item rating scale to measure medication adherence: further evidence for convergent validity. J Int Assoc Physicians AIDS Care. 2009;8(6):367–374. doi: 10.1177/1545109709352884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Josephs JS, Fleishman JA, Gaist P, Gebo KA. Use of complementary and alternative medicines among a multistate, multisite cohort of people living with HIV/AIDS. HIV Medicine. 2007;8(5):300–305. doi: 10.1111/j.1468-1293.2007.00474.x. for the HIV Research Network. [DOI] [PubMed] [Google Scholar]
- 7.Klos M, van de Venter M, Milne PJ, Traore HN, Meyer D, Oosthuizen V. In vitro anti-HIV activity of five selected South African medicinal plant extracts. J Ethnopharmacol. 2009;124(2):182–188. doi: 10.1016/j.jep.2009.04.043. [DOI] [PubMed] [Google Scholar]
- 8.Lamorde M, Tabuti JR, Obua C, Kukunda-Byobona C, Lanyero H, Byakika-Kibwika P, Bbosa GS, Lubega A, Ogwal-Okeng J, Ryan M, Waako PJ, Merry C. Medicinal plants used by traditional medicine practitioners for the treatment of HIV/AIDS and related conditions in Uganda. J Ethnopharmacol. 2010 May 5; doi: 10.1016/j.jep.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 9.Langlois-Klassen D, Kipp W, Jhangri GS, Rubaale T. Use of traditional herbal medicine by AIDS patients in Kabarole District, western Uganda. Am J Trop Med Hyg. 2007;77(4):757–763. [PubMed] [Google Scholar]
- 10.Littlewood RA, Vanable PA. Complementary and alternative medicine use among HIV-positive people: research synthesis and implications for HIV care. AIDS Care. 2008;20(8):1002–1018. doi: 10.1080/09540120701767216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu C, Yang Y, Gange SJ, Weber K, Sharp GB, Wilson TE, Levine A, Robison E, Goparaju L, Gandhi M, Merenstein D. Disclosure of complementary and alternative medicine use to health care providers among HIV-infected women. AIDS Patient Care STDS. 2009;23(11):965–971. doi: 10.1089/apc.2009.0134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maneesriwongul WL, Tulathong S, Fennie KP, Williams AB. Adherence to antiretroviral medication among HIV-positive patients in Thailand. J Acquir Immune Defic Syndr. 2006;43(Suppl 1):S119–S122. doi: 10.1097/01.qai.0000248346.79888.78. [DOI] [PubMed] [Google Scholar]
- 13.Malangu N. Self-reported use of traditional, complementary and over-the-counter medicines by HIV-infected patients on antiretroviral therapy in Pretoria, South Africa. Afr J Tradit Complement Altern Med. 2007;4(3):273–278. doi: 10.4314/ajtcam.v4i3.31219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mills E, Cooper C, Seely D, Kanfer I. African herbal medicines in the treatment of HIV: Hypoxis and Sutherlandia. An overview of evidence and pharmacology. Nutr J. 2005a;31(4):19. doi: 10.1186/1475-2891-4-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mills E, Foster BC, van Heeswijk R, Phillips E, Wilson K, Leonard B, Kosuge K, Kanfer I. Impact of African herbal medicines on antiretroviral metabolism. AIDS. 2005b;19(1):95–97. doi: 10.1097/00002030-200501030-00013. [DOI] [PubMed] [Google Scholar]
- 16.Owen-Smith A, Diclemente R, Wingood G. Complementary and alternative medicine use decreases adherence to HAART in HIV-positive women. AIDS Care. 2007;19(5):589–593. doi: 10.1080/09540120701203279. [DOI] [PubMed] [Google Scholar]
- 17.Owira PMO, Ojewole JAO. ‘African potato’ (Hypoxis hemerocallidea corm): a plant-medicine for modern and 21st century diseases of mankind? 2009 doi: 10.1002/ptr.2595. [DOI] [PubMed] [Google Scholar]
- 18.A review. Phytother Res. 23:147–152. doi: 10.1002/ptr.2595. [DOI] [PubMed] [Google Scholar]
- 19.Peltzer K, Friend-du Preez N, Ramlagan S, Fomundam H. Use of traditional, complementary and alternative medicine (TCAM) for HIV patients in KwaZulu-Natal, South Africa. BMC Public Health. 2008;24, 8(1):255. doi: 10.1186/14712458-8-255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peltzer K, Friend-du Preez N, Ramlagan S, Fomundam H, Anderson J. Traditional complementary and alternative medicine and antiretroviral treatment adherence among HIV patients in KwaZulu-Natal, South Africa. Afr J Tradit Complement Altern Med. 2010;7(2):125–137. [PMC free article] [PubMed] [Google Scholar]
- 21.Smit E, Oberholzer HM, Pretorius E. A review of immunomodulators with reference to Canova. Homeopathy. 2009;98(3):169–176. doi: 10.1016/j.homp.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 22.Theo A, Masebe T, Suzuki Y, Kikuchi H, Wada S, Obi CL, Bessong PO, Usuzawa M, Oshima Y, Hattori T. Peltophorum africanum, a traditional South African medicinal plant, contains an anti HIV-1 constituent, betulinic acid. Tohoku J Exp Med. 2009;217(2):93–99. doi: 10.1620/tjem.217.93. [DOI] [PubMed] [Google Scholar]


