Abstract
To mimic advanced stage of cancer development involving multi-organ metastasis, hydrodynamic delivery commonly used in gene transfer was explored for establishing concurrent tumors in the lung, liver and kidney using B16-F1 melanoma cells, 4T1 breast cells and Renca renal carcinoma cells, as a model. The procedure involves a rapid injection into a mouse tail-vein of serum-free medium, containing tumor cells in a volume equal to approximately 7–9% of body weight. Compared with the conventional tail vein injection of tumor cells resulting in tumor growth only in the lung, hydrodynamic injection is highly effective in establishing tumor growth in the liver, kidney and lung. All tumor cells examined including melanoma, breast metastatic and renal carcinoma cells showed significant tumor growth in these organs. These results suggest that the hydrodynamic delivery can be a valuable tool for modeling cancer in laboratory animals, especially in experimental mice.
Key words: hydrodynamic delivery, tumor models, cell delivery
Introduction
Cancer cells can spread to almost any part of the body via the blood, the lymphatics or both to generate metastases in various organs of cancer patients at the late stage. Thus, a multi-organ metastasis tumor model is an indispensible tool for studies aiming at elucidating the mechanism of metastasis and evaluating new strategy of treatment. Animal models of cancer, particularly mouse models, are commonly used to study tumor biology and to develop new approaches for treatment of human cancer. The most commonly used method for establishing a tumor model in animals is carcinogen-induced spontaneous tumor. The major advantage of spontaneous tumor development is that tumors developed closely mimic the tumor growth in patients. However, the drawback of this method is that it is time consuming and the resulted tumors are often heterogeneous in growth rate and properties, and unpredictable as well.1–3 Another method employed in cancer research is orthotopic or ectopic implantation of tumor cells into appropriate tissue. Although widely used, the implantation approach normally requires a surgical procedure in order to seed the cells in the internal organs such as the liver, kidney, spleen and pancreas.4–7
The hydrodynamic delivery method is a method developed for delivery of either DNA or siRNA for gene function and gene therapy studies in rodents.8–11 It involves a rapid tail-vein injection of a large volume (equal to 8–10% body weight) of siRNA/DNA solution into a mouse or rat to build high intravascular pressure, consequently, increasing endothelia permeability and facilitating intracellular delivery of nucleic acids. Following the initial report in 1999,8,9 this method has been widely used for assessing therapeutic function of genes and studying the activity of DNA sequence in regulating gene expression.10
Because of its high efficiency in delivering macromolecules into animals, we reasoned that this procedure might be applicable to cell delivery. Using tumor cells as a model, we demonstrated in this study that hydrodynamic tail-vein injection is effective in introducing tumor cells into multiple organs. Compared with the conventional tail-vein injection of tumor cells that resulted in tumor growth only in the lung, hydrodynamic injection resulted in tumor growth in the lung, liver and kidney. Data presented here suggest that hydrodynamic tail vein injection of tumor cells is a novel and effective procedure for simultaneous establishment of tumor growth in mouse lung, liver and kidneys.
Results
Simultaneous establishment of tumor growth in the lung, liver and kidney with B16-F1 cells.
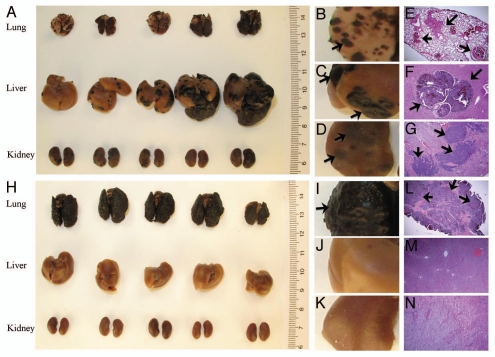
Melanoma is the most dangerous skin cancer derived from malignant melanocytes. It develops metastases at a later stage of tumor development in the lung (70–87%), liver (54–77%), kidney (35–48%) and other organs.12 Figure 1A shows that 15 d after hydrodynamic tail-vein injection of 106 B16-F1 melanoma cells, 100% mice (five mice) developed tumor nodules in the lung, liver and kidney (Fig. 1A–D). Tumor growth in these organs was also evidenced by H&E staining of the tissue sections from these organs (Fig. 1E–G). In contrast, conventional injection of the same number of cells in 100 µl generated tumor growth only in the lung (Fig. 1H–K), which agrees with the result of H&E staining (Fig. 1L–N). Slow infusion of the same number of cells in volume equal to that of hydrodynamic injection was performed as an additional control. Like conventional tail-vein injection in small volume, slow infusion of tumor cells in large volume resulted in tumor growth only in the lung (data not shown), confirming that tumor growth in the liver and the kidney is caused by the combined effect of large volume and rapid injection.
Figure 1.
Simultaneous establishment of tumor growth in the lung, liver and kidney with B16-F1 cells. B16-F1 melanoma cells in serum-free medium were tail vein injected following either the hydrodynamic (7.5% body weight in volume, 5 sec injection time) (A–G) or conventional (H–N) method at a dose 1 × 106 cells/mouse. Mice were sacrificed 15 d after the injection, and the lungs, livers and kidneys were harvested and fixed immediately in 10% neutral buffered formalin. Pictures were taken using a Canon G9 digital camera. Five micrometer tissue sections were made for each organ and H&E staining was performed. Arrows point to the tumor sites.
Simultaneous establishment of tumor growth in the lung, liver and kidney with 4T1 cells.
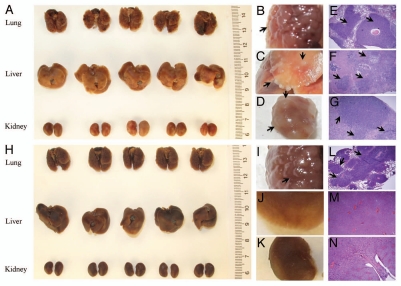
Additional cell line was used to confirm the hydrodynamic effect on establishing tumor growth in multiple organs. The 4T1 cells that are commonly used as a model for stage IV breast cancer13–15 were hydrodynamically injected into Balb/c mice at a dose of 5 × 105 cells/mouse. Similar to B16-F1 cells, tumor growth in the lung, liver and kidney was seen in all mice with hydrodynamic injection (Fig. 2A–G), compared with tumor growth only in the lung when conventional injection was performed (Fig. 2H–N).
Figure 2.
Simultaneous establishment of tumor growth in the lung, liver and kidney with 4T1 cells. 4T1 breast tumor cells were injected into the tail-vein following either the hydrodynamic procedure (A–G) or conventional method (H–N) at a dose of 5 × 105 cells/mouse. Animals were sacrificed 12 d after the injection, and the lung, liver and kidneys were collected and immediately fixed in 10% neutral buffered formalin. Tumor growth in each organ was documented using a Canon G9 digital camera. (E–G) represent the H&E staining of the tissue sections from the lung, liver and kidney of the hydrodynamically injected animals. (L–N) represent H&E staining of the tissue sections from the lung, liver and kidney of animals receiving conventional injection. Arrows indicate the tumor sites.
Simultaneous establishment of tumor growth in the lung, liver and kidney with Renca cells.
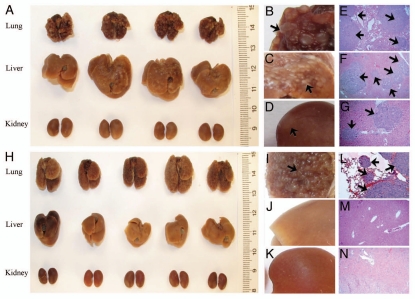
Renca cells are renal adenocarcinoma cells that are commonly used for studies of kidney-liver, kidney-lung metastasis.16,17 Results in Figure 3A show 100% of apparent tumor growth (Fig. 3A–C) in the lung and liver that was confirmed by histochemistry (Fig. 3E and F) after hydrodynamic delivery of 5 × 105 Renca cells into each Balb/c mouse. Tumor growth in kidneys was not apparent from the exterior (Fig. 3D), but readily visible in H&E staining of tissue sections (Fig. 3G). Interestingly, though Renca cells were originally derived from a spontaneous renal carcinoma in Balb/c mice, they grew better in the lung and liver after hydrodynamic delivery. Again, conventional tail vein injection of the same number of cells resulted in tumor growth only in the lung (Fig. 3H–N).
Figure 3.
Simultaneous establishment of tumor growth in the lung, liver and kidney with Renca cells. Renca cells suspended in serum free medium were tail vein injected following the hydrodynamic (A–G) (9% body weight in volume, 5 sec) or conventional (H–N) (100 µl, 5 sec) procedure at a dose of 5 × 105 cells/mouse. Mice were sacrificed 20 d after tumor injection, and the lungs, livers and kidneys were collected and immediately fixed in 10% neutral buffered formalin. Pictures were taken using a Canon G9 digital camera. Arrows point the tumor sites.
Discussion
Direct injection of tumor cells into the target tissue or nearby organs with vascular connection is broadly used to establish tumor growth in mice. Good examples of intra-tissue tumor injection include establishing brain tumor by injection of tumor cells into the carotid artery;18 liver tumor by intra-splenic, intra-portal or intra-hepatic cell injections; and lung tumor by lateral tail vein injection. In the current study, we demonstrated that simultaneous tumor growth in mouse lung, liver and kidneys can be achieved by a rapid tail-vein injection of tumor cells in a volume equal to 7–9% of body weight. Compared with the conventional tail vein injection that normally results in tumor growth in the lung, hydrodynamic injection of three different types of cancer cells results in 100% simultaneous tumor growth in mouse lung, liver and kidney (Figs. 1–3).
The effect of hydrodynamic cell delivery on tumor growth in the lung, liver and kidney demonstrated in this study can be explained by the fact that a rapid injection of large volume solution into the mouse tail-vein generates a rapid accumulation of solution in the inferior vena cava due to limited cardiac output. The accumulated solution increases the hydrodynamic pressure in this venous section and drives the cell-containing solution into the liver and kidney in retrograde through the hepatic and renal vein, respectively. Consequently, tumor cells are delivered into the liver and kidneys. While some tumor cells are trapped in the liver and kidneys, the remaining tumor cells enter the heart and are then trapped in the lung due to the endothelial bed effect. In the absence of the hydrodynamic pressure, as in the case of the conventional and slow infusion, all injected cells enter the inferior vena cava and are carried into the heart and then to the lung where they are trapped due to its unique vasculature and low blood flow rate. If no cells escape from the lung, tumor growth should not be seen in other organs.
The safety of hydrodynamic delivery into mice via the tail-vein has been well established.8 However, specific attention is needed when a high number of cells is used for hydrodynamic delivery, as serious lung embolism could occur.
In summary, results presented in this study suggest that hydrodynamic delivery from the tail vein is a simple, effective and convenient method to establish simultaneous tumor growth in mouse lung, liver and kidney. This method could be extremely useful for studies dealing with environmental influences on tumor growth. Tumor growth in the lung, liver and kidney can be established from the same group of cells, while the difference in tumor growth rate in these organs could suggest the difference of how the tumor cells interact with lung, liver or kidney cells. At the same time, this procedure provides a great opportunity to study whether/how tumor cells in different organs respond to a drug treatment. Our success in establishing tumor growth in different organs also indicates that hydrodynamic delivery may also be applicable to the delivery of stem cells for basic and clinical research.
Materials and Methods
Cells and mice.
B16-F1 cells (mouse melanoma), 4T1 cells (mouse breast cancer), and Renca (mouse renal carcinoma) cells were from ATCC. B16-F1 and Renca cells were cultured in DMEM with 10% FBS and 1% penicillin/streptomycin. 4T1 cells were cultured in RPMI 1640 with 10% FBS and 1% penicillin/streptomycin. Female Balb/c (6–8 weeks old, 18–22 g) and C57BL/6 (6–8 weeks old, 20–22 g) mice were purchased from Charles River Laboratories and housed in a pathogen-free environment in the Animal Facility of the University of Pittsburgh.
Preparation of single cell suspension.
About 2 × 106 B16-F1 cells were seeded into a 100 × 20 mm cell culture dish and allowed to grow for 24 h in serum-containing medium. After removing the medium, each plate was treated with 3 ml of Trypsin/EDTA solution (0.25% Trypsin, 2.21 mM EDTA) at room temperature for 7 min. Cell suspension was mixed with 5 ml of serum containing medium and gently pipetted a few times. The mixture was passed through a membrane filter (45 µm in pore size) and centrifuged for 5 min at room temperature at 1,500 rpm. Cell pellet was re-suspended in 7.5 ml of RPMI-1640 serum-free medium and passed through a membrane filter again. After centrifugation at the same speed, cell pellet was re-suspended in 3 ml of serum free medium and passed through a membrane filter for the third time. Cell concentration was determined using a hemocytometer and diluted to the desirable concentration. Same procedure was used for 4T1 and Renca cells with the exception that trypsinization time for 4T1 cells was 15 min at room temperature and 3 min for Renca cells at 37°C.
Hydrodynamic cell delivery.
Cell suspension in serum-free medium was injected into the tail vein in 5 sec with a volume equal to 7.5% body weight into a C57BL/6 mouse for B16-F1 cells. The volume used for 4T1 and Renca cells was 9% body weight of a Balb/c mouse. A plastic syringe (3 ml) and 27G1/2 needle were employed for injection. For control animals, the same number of cells were either injected by conventional method (100 µl, ∼5 sec) or infused in large volume (9% body weight) into the mouse tail vein in about 1 min.
Histology analysis.
Tumor-bearing mice were sacrificed with CO2 when they showed signs of physical stress, such as respiratory difficulty or weight loss according to the IACUC guidance at the University of Pittsburgh. Main organs were removed from the animals, fixed in 10% neutral-buffered formaldehyde for 48 h at room temperature and photographed. Paraffin sections (5 µm) of the lung, liver and kidney were prepared using a standard histological method19 and stained with Hematoxylin and Eosin (H&E) according to manufacturer's instruction (BBS Biochemical).
Acknowledgments
This study is supported in part by NIH grants RO1 EB007357-02S1, RO1 EB007357 and RO1 HL098295.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Trosko JE, Chang CC. Environmental carcinogenesis: an integrative model. Q Rev Biol. 1978;53:115–141. doi: 10.1086/410451. [DOI] [PubMed] [Google Scholar]
- 2.Yuspa SH, Hennings H, Saffiotti U. Cutaneous chemical carcinogenesis: past, present and future. J Invest Dermatol. 1976;67:199–208. doi: 10.1111/1523-747.ep12513040. [DOI] [PubMed] [Google Scholar]
- 3.Ottewell PD, Coleman RE, Holen I. From genetic abnormality to metastases: murine models of breast cancer and their use in the development of anticancer therapies. Breast Cancer Res Treat. 2006;96:101–113. doi: 10.1007/s10549-005-9067-x. [DOI] [PubMed] [Google Scholar]
- 4.Tan MH, Holyoke ED, Goldrosen MH. Murine colon adenocarcinoma: syngeneic orthotopic transplantation and subsequent hepatic metastases. J Natl Cancer Inst. 1977;59:1537–1544. doi: 10.1093/jnci/59.5.1537. [DOI] [PubMed] [Google Scholar]
- 5.Manzotti C, Audisio RA, Pratesi G. Importance of orthotopic implantation for human tumors as model systems: relevance to metastasis and invasion. Clin Exp Metastasis. 1993;11:5–14. doi: 10.1007/BF00880061. [DOI] [PubMed] [Google Scholar]
- 6.Vezeridis MP, Doremus CM, Tibbetts LM, Tzanakakis G, Jackson BT. Invasion and metastasis following orthotopic transplantation of human pancreatic cancer in the nude mouse. J Surg Oncol. 1989;40:61–64. doi: 10.1002/jso.2930400412. [DOI] [PubMed] [Google Scholar]
- 7.Lafreniere R, Rosenberg SA. Successful immunotherapy of murine experimental hepatic metastases with lymphokine-activated killer cells and recombinant interleukin 2. Cancer Res. 1985;45:3735–3741. [PubMed] [Google Scholar]
- 8.Liu F, Song Y, Liu D. Hydrodynamics-based transfection in animals by systemic administration of plasmid DNA. Gene Ther. 1999;6:1258–1266. doi: 10.1038/sj.gt.3300947. [DOI] [PubMed] [Google Scholar]
- 9.Zhang G, Budker V, Wolff VA. High levels of foreign gene expression in hepatocytes after tail vein injections of naked plasmid DNA. Hum Gene Ther. 1999;10:1735–1737. doi: 10.1089/10430349950017734. [DOI] [PubMed] [Google Scholar]
- 10.Suda T, Liu D. Hydrodynamic Gene Delivery: Its principles and applications. Mol Ther. 2007;15:2063–2069. doi: 10.1038/sj.mt.6300314. [DOI] [PubMed] [Google Scholar]
- 11.Zhou T, Kamimura K, Zhang G, Liu D. Intracellular gene transfer in rats by tail vein injection of plasmid DNA. AAPS J. 2010;12:692–698. doi: 10.1208/s12248-010-9231-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meyers ML, Balch CM. Diagnosis and treatment of metastatic melanoma. In: Balch CM, Houghton AN, Sober AJ, Soong SJ, editors. Cutaneous Melanoma. Vol. 3. St. Louis: Quality Medical Publishing; 1998. p. 329. [Google Scholar]
- 13.Aslakson CJ, Miller FR. Selective events in the metastatic process defined by analysis of the sequential dissemination of subpopulations of a mouse mammary tumor. Cancer Res. 1992;52:1399–1405. [PubMed] [Google Scholar]
- 14.Dexter DL, Kowalski HM, Blazar BA, Fligiel Z, Vogel R, Heppner GH. Heterogeneity of tumor cells from a single mouse mammary tumor. Cancer Res. 1978;38:3174–3181. [PubMed] [Google Scholar]
- 15.Heppner GH, Miller FR, Shekhar PM. Nontransgenic models of breast cancer. Breast Cancer Res. 2000;2:331–334. doi: 10.1186/bcr77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yoon SS, Eto H, Lin C, Lin CM, Nakamura H, Pawlik TM, et al. Mouse endostatin inhibits the formation of lung and liver metastases. Cancer Res. 1999;59:6251–6256. [PubMed] [Google Scholar]
- 17.Verheul HM, Hammers H, Van Erp K, Wei Y, Sanni T, Salumbides B, et al. Vascular endothelial growth factor trap blocks tumor growth, metastasis formation and vascular leakage in an orthotopic murine renal cell cancer model. Clin Cancer Res. 2007;13:4201–4208. doi: 10.1158/1078-0432. CCR-06-2553. [DOI] [PubMed] [Google Scholar]
- 18.Miner KM, Kawaguchi T, Uba GW, Nicolson GL. Clonal drift of cell surface, melanogenic and experimental metastatic properties of in vivo-selected, brain meninges-colonizing murine B16 melanoma. Cancer Res. 1982;42:4631–4638. [PubMed] [Google Scholar]
- 19.Dempster WT. The mechanics of paraffin sectioning by the microtome. Anat Rec. 1942;84:241–267. doi: 10.1002/ar.1090840303. [DOI] [Google Scholar]